Higher-level only
The rate of the substitution reactions is influenced by the identity of the leaving group.
Syllabus ref: R3.4.10Reactivity 3.4.10 - The rate of the substitution reactions is influenced by the identity of the leaving group. (HL)
- Predict and explain the relative rates of the substitution reactions for different halogenoalkanes. Different halogenoalkanes should include RCl, RBr, RI.
Guidance
- The roles of the solvent and the reaction mechanism on the rate will not be assessed.
Tools and links
- Structure 3.1 - Why is the iodide ion a better leaving group than the chloride ion?
Factors affecting reaction rate
The rate of reaction is dependent on two factors:
- Whether the haloalkane is primary, secondary of tertiary
- The actual halogen atoms attached to the alkyl chain
Mechanism
Tertiary haloakanes react via an sN1 mechanism that has a much lower activation energy than the sN2 mechanism with the high energy transition state. Hence tertiary haloalkanes react faster then secondary, which in turn react faster than primary.
The leaving group
The atomic and ionic radius of the halogens increases descending the group.
iodine >> bromine >> chlorine
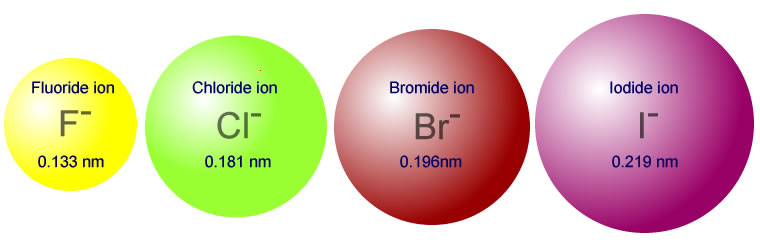
This means that the bond formed between carbon and iodine is longer and consequently weaker than the bond formed between bromine and carbon, which in turn is weaker than the bond formed between chlorine and carbon. It is therefore easier to break off an iodide ion than a bromide or chloride ion. The rate of reaction is in the order:
iodoalkanes >> bromoalkanes >> chloroalkanes
A second factor is the relative stability of the leaving group. Group 17 ions have a full outer shell and are very stable, although iodide ions are reducing agents.
They are all very stable as leaving groups, particularly in polar solutions.
Note: Students are no longer expected to know the effects of solvent on reaction rate
Stereospecificity
Mechanisms that proceed via an sN2 mechanism cause inversion at the carbon atom that is attacked by the nucleophile.
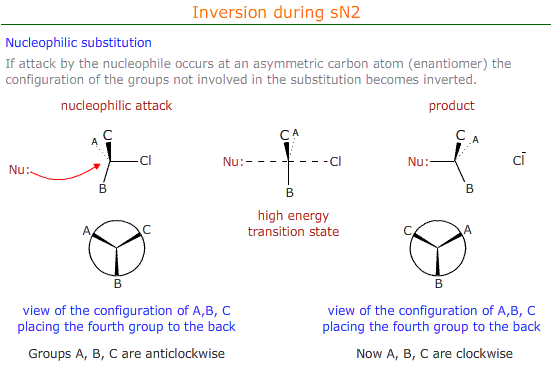
This has consequences for the optical properties and the designation of the configuration (R or S) of the stereoisomer.
If the enantiomer has an "R" conformation before nucleophilic substitution, as determined by CIP priority rules, then the product of the substitution has an "S" configuration