Higher-level only
Alkenes are hydrocarbons that contain an alkenyl functional group. They are manufactured industrially by cracking hydrocarbons and are much more useful than alkanes chemically, as the double bond makes them reactive towards many other species. They readily undergo electrophilic addition reactions.
Syllabus ref: R3.4.11Reactivity 3.4.11 - Alkenes readily undergo electrophilic addition reactions. (HL)
- Describe and explain the mechanisms of the reactions between symmetrical alkenes and halogens, water and hydrogen halides.
Guidance
Tools and links
Structural features
Alkenes are organic compounds that contain a double carbon-carbon (alkenyl) bond. They are said to be 'unsaturated' in that they can add more hydrogen atoms to the hydrocarbon skeleton.
The double carbon-carbon bond is a region of high electron density, with one pair of electrons in a 'pi' orbital, which can be attacked by reagents containing a positive or partial positive charge. This makes alkenes reactive and useful as building blocks for organic synthesis.
Naming alkenes
The position of the double bond is numbered using the carbon with the smallest number in the chain. If there is branching the chain is chosen that includes the double bond.
Example: Name the following alkene:
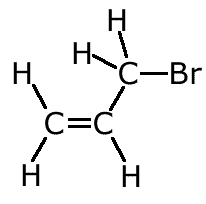
The longest chain with the double bond has three carbon atoms. The chain is numbered to keep the double bond with the lowest number. The bromine, therefore is on carbon number 3. Hence 3-bromopropene
Electrophilic addition reactions
Alkenes can form addition products with other molecules by 'opening' the double bond and using the electrons to form bonds at each carbon atom. This is known as addition.
The process is stimulated by electrophilic attack by the reagent, and for this reason is called electrophilic addition.
Alkenes react with halogens making disubstituted halogenoalkanes
ethene + bromine → 1,2-dibromoethane
C2H4 + Br2 → C2H4Br2
Alkenes react with interhalogens making disubstituted halogenoalkanes
ethene + iodine monochloride → 1-chloro-2-iodoethane
C2H4 + ICl → CH2ClCH2I
Alkenes react with hydrogen halides making halogenoalkanes:
ethene + hydrogen bromide → bromoethane
C2H4 + HBr → C2H5Br
Alkenes react with steam in the presence of a catalyst making alcohols:
ethene + steam → ethanol
C2H4 + H2O → C2H5OH
Alkenes react with hydrogen in the presence of a Ni catalyst at 150ºC making alkanes:
ethene + hydrogen → ethane
C2H4 + H2 → C2H6
Although it may seem pointless making an unreactive alkane from its useful alkane, this reaction is important in synthesis, as it allows control of the degree of unsaturation of long chain compounds that have several double bonds, such as the vegetable oils.
Changing double bonds to single bonds, via hydrogenation, increases the melting point of a vegetable oil, an essential step in the production of margarine.
The mechanism of electrophilic addition
An electrophile is an electron-deficient species that can accept electron pairs from a nucleophile. Electrophiles are Lewis acids. Deduction of the mechanism of the electrophilic addition reactions of alkenes with halogens/interhalogens and hydrogen halides.
Step 1: Attack by the electrophile - the pair of electrons in the pi orbital of the alkenyl group moves to attach the partially positive side of the polarised electrophile forming a carbo-cation (positive) intermediate and breaking the bond in the electrophile heterolytically.
Step 2: The negative ion remaining from the heterolytic fission of the electrophile then attacks the carbon atom holding the positive charge on the intermediate.
Mechanism - electrophilic addition
Polymerisation
Polymers are large molecules that consist of many repeating units (monomers)
They may be categorised as addition polymers or condensation polymers
An addition polymer is one that is formed by addition reactions of alkenes to one another. The individual alkene molecule is the monomer and the product of joining many monomers together is called the polymer.
Alkenes can polymerise in the presence of suitable catalysts. Originally this was carried out using high pressure and an oxygen catalyst, but nowadays there are more efficient catalysts containing titanium compounds that can make better polymeric products (Zeigler-Natta catalysts).
C2H4 → -[C2H4]n-
ethene → poly(ethene)
By changing the substituents around the double bond, a large variety of different polymers can be produced, each one with different properties.
C6H5CHCH2 → -[C6H5CHCH2]n-
phenylethene (styrene) → poly(styrene)
The properties of addition polymers
The specific properties depend largely on groups that are attached to the hydrocarbon chain and also any other materials that are incorporated into the structure when the polymer is manufactured.
The simplest polymer, poly(ethene), also known as polythene, is a soft, insoluble substance used for plastic bags.
Addition polymers may be moulded into shape at elevated temperatures, when they soften as the structure weakens. The large molecules are held together by Strong London dispersion forces. The more regular the arrangement of these molecules, the stronger the intermolecular forces.
High density polymers have very regular structures and may be rigid and hard. A typical example would be high density poly(ethene), HDPE.
Worked examples
Q1032-01 The presence of a carbon-carbon double bond in an organic molecule:- results in a linkage between carbon atoms that has a much smaller bond energy that that of single carbon to carbon bonds.
- results in greater reactivity, often with addition of two atoms to the two carbon atoms of the bond.
- makes the molecule much more polar and therefore much more soluble in water.
- allows internal molecular rotations so that there are no isomers with the same formula possible.
|
The double bond overall is stronger than a single carbon bond, but little energy is required to break the pi system and open the double bond. Hence a double bond results in greater reactivity, often with addition of two atoms to the two carbon atoms of the bond. Response B |
Q1032-02 Which product is formed by the reaction between CH2CH2 and HBr
- CH3CH2Br
- CH2CHBr
- BrCHCHBr
- CH3CH2Br2
|
This is a hydrohalogenation addition reaction producing CH3CH2Br |
Q1032-03 What type of reaction does the equation below represent?
CH2=CH2 + Br2 → BrCH2CH2Br
- Substitution
- Condensation
- Reduction
- Addition
|
This is an addition reaction, or more specifically, electrophilic addition. |
Q1032-04 Which of the following statements about single and double bonds between two carbon atoms is (are) correct?
- I. double bonds are stronger than single bonds
- II. double bonds are more reactive than single bonds
- I only
- II only
- Both I and II
- Neither I nor II
|
Both of the statements are correct, response C |
Q1032-05 Name the type of polymerisation reaction that C3H6 undergoes and draw the structure of a section of the polymer chain formed from three monomer molecules.
Answer
|
C3H6 is propene and polymerises by addition polymerisation.
|
Q1032-06 A gaseous alkane and a gaseous alkene are treated separately in the following ways. Which treatment will distinguish between them?
- They are ignited in excess oxygen
- They are passed over heated copper
- They are bubbled through an aqueous solution of bromine
- They are bubbled through an aqueous solution of propanal
|
Bubbling both gases through aqueous bromine can distinguish them. In the case of the alkene the orange bromine solution becomes decolourised. There is no change in the case of the alkane. |
Q1032-07 Polymers formed from monomers with the general formula H2C=CHX
- Have the same percentage of carbon as the monomer
- Are produced by substitution reactions
- Contain C=C bonds
- Are more reactive than the monomer
|
As the empirical formula of the alkene and the polymer is the same they both have the same percentage composition. |
Q1032-08 Which compound is most likely to be a starting material for a common polymer?
- CH3CH2CH3
- CH3CH2OH
- CH3CHCH2
- C6H5CH3
|
Addition polymers are made from alkenes. The only alkene among the choices is CH3CHCH2 |
Q1032-09 In the reaction below state the type of reaction and identify the reagent and conditions needed.
C2H4 → CH3CH2OH
Answer|
This is an addition reaction. The reagent is water in the form of steam. The gases must be passed over a phosphoric acid catalyst at 300ºC. |
Q1032-10 C2H4 can be converted into one of the compounds below in a single step reaction
- C2H3Cl
- C2H4Cl2
Identify the compound that can be formed directly from C2H4
|
Addition of chlorine to ethene produces the compound C2H4Cl2 by addition. |