Standard level
The term "heterolytic fission" is made up of of two parts, "hetero + litic" = uneven cleavage into two different parts, and fission = breaking apart.
Syllabus ref: R3.4.3Reactivity 3.4.3 - Heterolytic fission is the breakage of a covalent bond when both bonding electrons remain with one of the two fragments formed.
- Explain, with equations, the formation of ions by heterolytic fission.
Guidance
- Curly arrows should be used to show the movement of electron pairs during reactions.
Tools and links
- Reactivity 3.3 - What is the difference between the bond-breaking that forms a radical and the bond-breaking that occurs in nucleophilic substitution reactions?
The covalent bond
This is fundamentally a shared pair of electrons holding two nuclear centres together by electrostatic attraction.
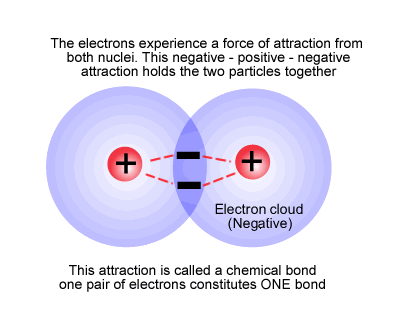
The relative positions of the two atoms is determined by the balance of attractive and repulsive forces that constitutes a chemical potential energy minimum.
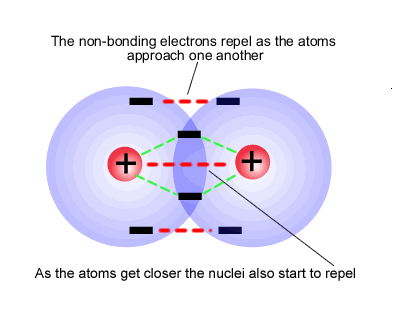
We call this "ideal" potential energy minimum distance the "bond length". It is different for each different pair of atoms. Carbon-carbon single bond length is 154 pm.
Bond cleavage
Logically there are two different things that can happen to the electron pair when the bond breaks.
- 1 The electron pair stays with one of the atoms only.
- 2 Each atom gets one of the electrons.
The first type of cleavage (bond breaking) is called heterolytic fission, when one atom gets both of the electrons.
Consequences of heterolytic fission
Each electron carries a negative charge. While the bond is maintained, the electron pair is essentially providing one negative charge to each of the bonded atom, cancelling out one of the nucleus positive charges.
However, if both electrons go to one atom, then that atom gains an extra negative charge and the other atom loses a negative charge, making a positive particle.
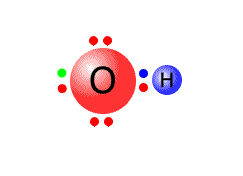
For example, a water molecule has the Lewis structure:
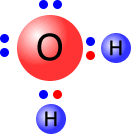
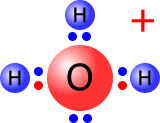
Water is a neutral species, as all of the positive charges in the nuclei are balanced by all of the negative charges of the electrons. But if one of the OH bonds breaks heterolytically, with the electron pair remaining on the oxygen atom then we get two particles, each of which carries an electrical charge, the hydroxide ion, OH- and the hydrogen ion, H+.
Dissociation of water
H2O ⇋ H+ + OH-