Higher-level only
A Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor. (HL)
Syllabus ref: R3.4.6Reactivity 3.4.6 - A Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor. (HL)
- Apply Lewis acid–base theory to inorganic and organic chemistry to identify the role of the reacting species.
Guidance
Tools and links
- Reactivity 3.1 - What is the relationship between Brønsted–Lowry acids and bases and Lewis acids and bases?
Lewis acids
Lewis acids are electron pair acceptors. The electron pair can originate from any species, ion or molecule that has a lone-pair of electrons not involved in bonding.
Examples of Lewis acids include aluminium trichloride, transition metal ions (and in some cases, atoms), polarised organic molecules and organic or inorganic molecules in which polarisation can be induced.
The bromination of benzene
C6H6 + Br2 → C6H5Br + HBr
Bromine molecules are non-polar, but in the presence of an iron catalyst an iron(III) bromide intermediate is formed "in-situ" which is a Lewis acid in the form of FeBr4-Br+. It is the positive bromine atom that acts as the electrophile drawing an electron pair from the benzene delocalised electron ring. This starts the reaction mechanism.
Lewis bases
Lewis bases are species with lone pairs of electrons that can be coordinated to a species that is electron deficient in some way.
In transition metal complex formation, all ligands are electron pair donors, Lewis bases, coordinating the electron pair (or in some cases more that one electron pair) to the transition metal ion.
In organic chemistry, all nucleophiles are Lewis bases, seeking positive or partially positive atoms to which their electron pair can coordinate.
Lewis acid-base reactions
Clearly, with one species accepting an electron pair and another species donating an electron pair, Lewis acids react easily with Lewis bases.
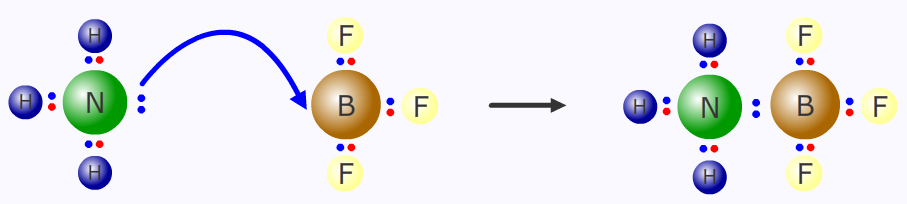
The ammonia molecule is a Lewis base with its lone pair of electrons and the boron trifluoride molecule is a Lewis acid with its electron deficient outer shell. There is perfect symbiosis!
A Lewis acid - base reaction
NH3 + BF3 → NH3BF3
The convention for showing the movement of a pair of electrons is the "curly arrow". The arrow should begin at the origin of the lone pair and point to the electrophilic (Lewis acid) atom.