Standard level
The particulate theory of matter first was postulated by Democritus in the fifth century BC. He decided through inductive thought that all matter was composed of tiny indivisible particles, which he called "atomos".
The theory was re-discovered by John Dalton at the end of the eighteenth century AD in the course of his investigations into gases.
Historically, this is all very interesting, but what you actually need to know is the motion, forces and distance between the particles in solids, liquids and gases.
Syllabus ref: S1.1.2Structure 1.1.2 - The kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of state.
- Distinguish the different states of matter.
- Use state symbols (s, l, g, and aq) in chemical equations.
Guidance
- Names of the changes of state should be covered: melting, freezing, vaporization (evaporation and boiling), condensation, sublimation and deposition.
Tools and links
- Structure 2.4 - Why are some substances solid, while others are fluid under standard conditions?
- Structure 2 (all)
- Reactivity 1.2 - Why are some changes of state endothermic and some exothermic?
The particulate theory of matter
All of the available evidence points to the fact that all matter is made of particles and that they are in constant motion. The observable properties of matter are a function of these particles and the way that they are joined together.
What exactly is this "evidence"?
- Diffusion of gases
- Brownian motion
- Dissolution of substances in liquids
- Compressibility of gases
The particles of solids and liquids must be close together as both states of matter are not easily compressible, while gas particles are far apart as gases are highly compressible.
The kinetic theory describes the motion of the particles in solids, liquids and gases. At any temperature above absolute zero the particles have energy and this is expressed as motion (kinetic energy).
The energy is distributed over all of the particles in a random manner. This results in a statistical sharing out of the energy, called a Maxwell-Boltzmann distribution:
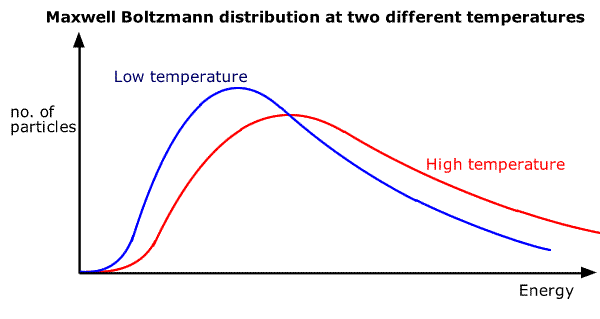
As shown in the above diagram, the shape of the distribution is basically the same as the temperature changes with very few particles having high or low energy, while the median energy moves up as the temperature increases. The average (mean) energy of the particles is always proportional to the temperature of the sample.
The way that particles are allowed to move is called their degree of freedom. The particles in a solid are only able to vibrate about fixed positions within a structure, while the particles in a liquid are able to vibrate, rotate and displace (translate) to a limited degree.
Particles in a gas have freedom of motion in terms of vibration rotation and translation.
At room temperature gas particles move very fast and collide very often. As they all move in different directions with different speeds the square root of mean square speed is used as a measure of their average velocity.
The quantity root mean square speed is defined as vrms = √(3RT/Mr), Where
- Mr is the molar mass in kilograms per mole
- T is the absolute temperature
- R is the Universal gas constant
For example, the root mean square speed of ammonia at room temperature = √(3 * 8.314 * 298/0.017) = 660 metres per second
The distance between particles
Solids and liquids are very difficult to compress, while gases are very easy to compress. This shows that the particles in solids and liquids are very close together, while the particles in gases are very far apart.
However, as the gas particles are travelling very fast, they collide very frequently. The average distance between collisions is called the "mean free path" and can be calculated from first principles.
The characteristics of the particles and their motion on a particulate scale defined the macroscopic physical properties.
External video resources
The kinetic theory of matter: Richard Thornley