Standard level
"Big fleas have little fleas upon their backs to bite them, and little fleas have lesser fleas, and so ad infinitum". Jonathan Swift.
Although atoms are said to be the smallest building blocks of matter, they themselves consist of smaller 'sub-atomic' particles. So what are sub-atomic particles made from? That, happily, remains the domain of the physicist and, as such, has no place on this platform!
Syllabus ref: S1.2.1Structure 1.2.1 - Atoms contain a positively charged, dense nucleus composed of protons and neutrons (nucleons). Negatively charged electrons occupy the space outside the nucleus.
- Use the nuclear symbol ZXA to deduce the number of protons, neutrons and electrons in atoms and ions.
Guidance
- Relative masses and charges of the subatomic particles should be known; actual values are given in the data booklet. The mass of the electron can be considered negligible.
Tools and links
- Structure 1.3 - What determines the different chemical properties of atoms?
- Structure 3.1 - How does the atomic number relate to the position of an element in the periodic table?
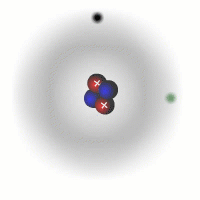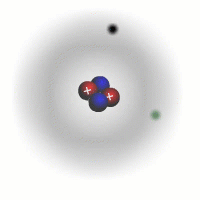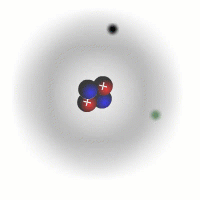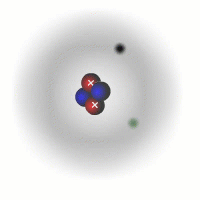
Evidence for sub-atomic particles
Atoms are the fundamental building blocks of matter. However, even atoms are constructed of smaller, sub-atomic particles. It was originally thought that the atoms were small indivisible particles (atomos = indivisible), however a paradigm shift occured with the discovery that the atoms themselves have a sub-structure.
Evidence for this emerged though the experiments conducted in the nineteenth century on cathode rays, culminating in the experiments of Rutherford (the nuclear atom) and Chadwick (neutrons) in the early 20th century.
It is now known that even these sub atomic particles themselves have an even deeper sub-structure - the quarks!
The fundamental sub-atomic particles are protons, neutrons and electrons. The protons and neutrons are held together by strong nuclear binding forces in the nucleus of the atom. The electrons may be considered to be tiny particles that exist in regions of space known as orbitals around the atom.
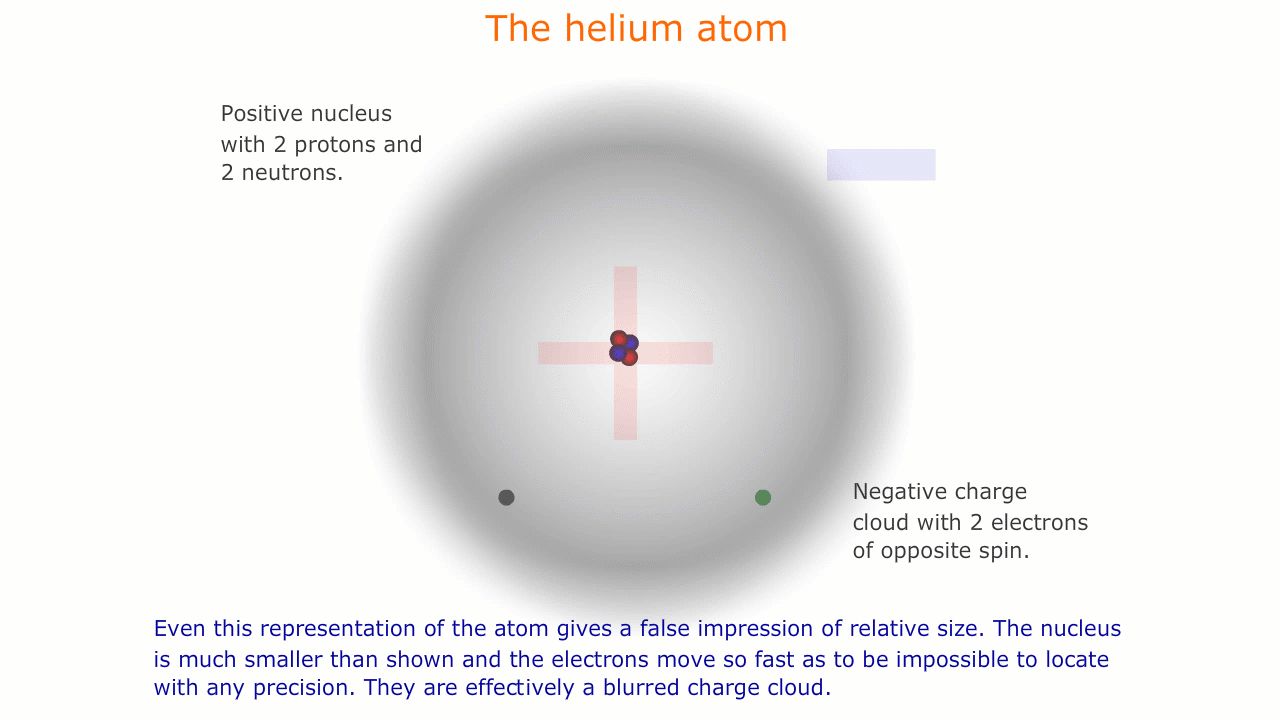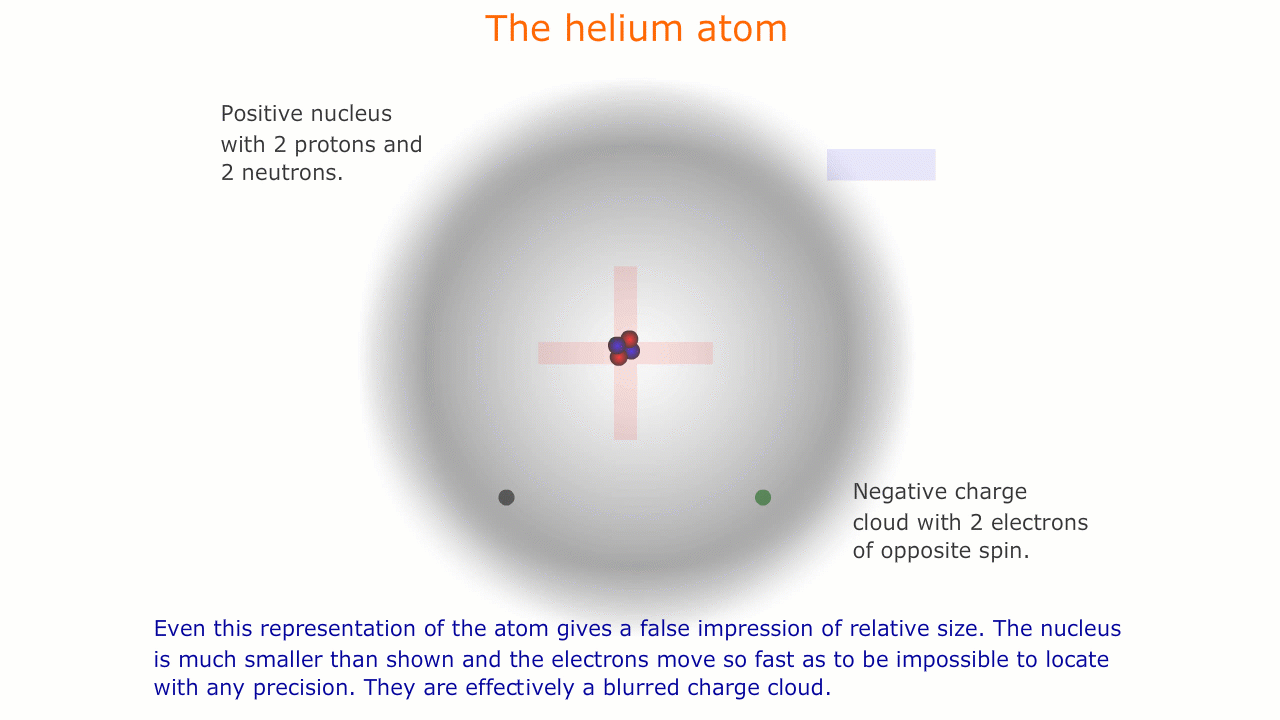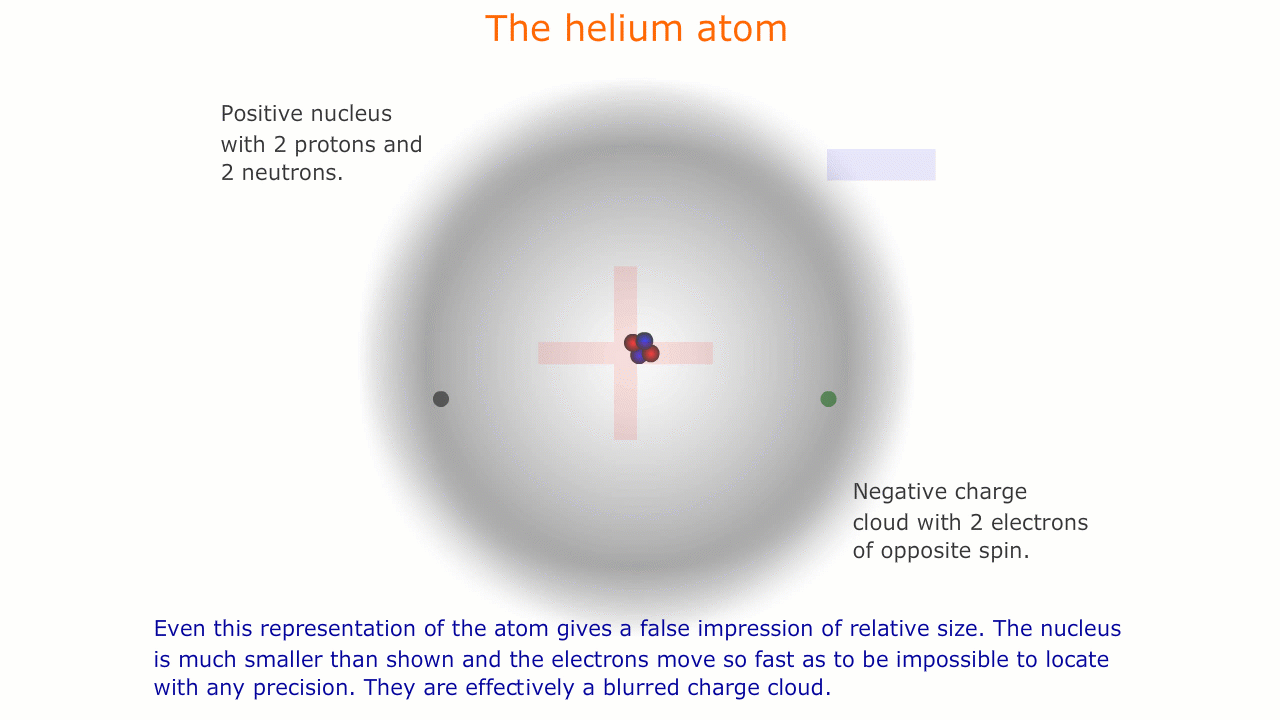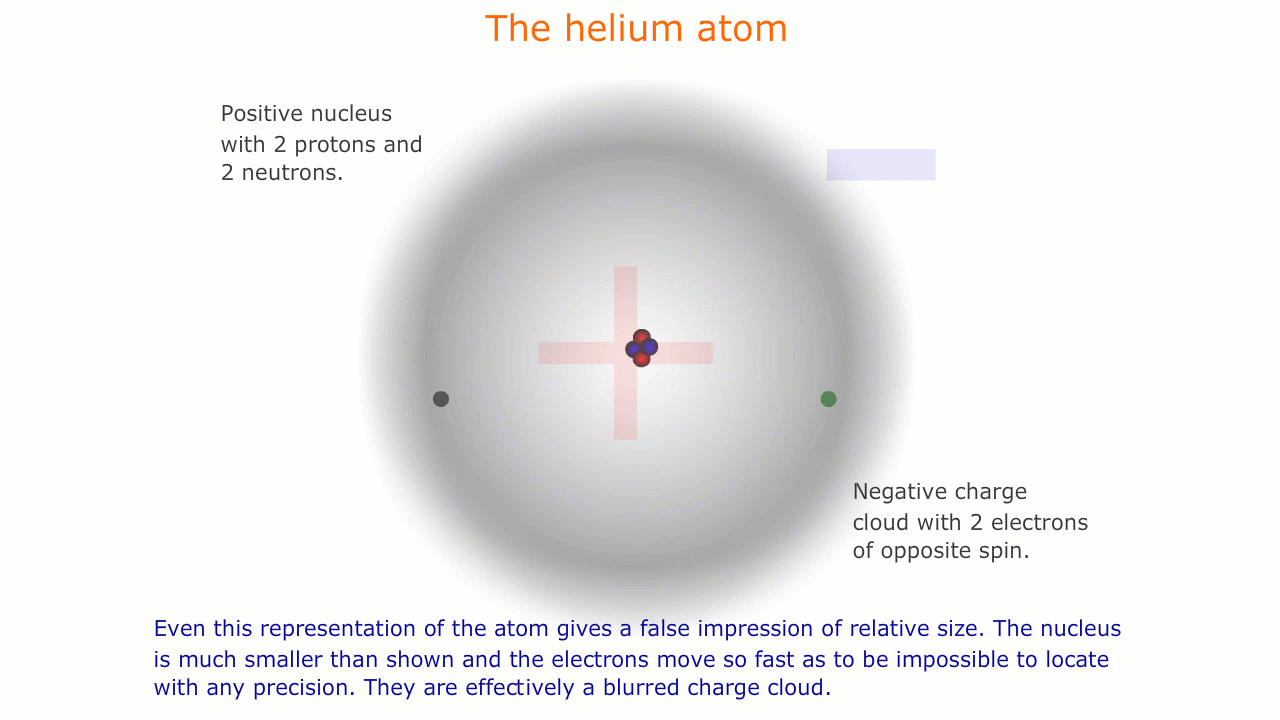
This model of the atom is precisely that, a model. It is impossible to see atoms and, in order to be able to describe their properties, we use models representing this microscopic world that is invisible to us.
Similarly, the sub-atomic world is a strange place with unusual forces acting over infintesimally small distances. The rules of behaviour that govern the macroscopic world often break down in this strange environment, and it is important to understand that our representations and models are necessarily limited here.
The picture of the atom above is easy to discuss and is comfortably familiar. However, if you consider the actual dimensions of an atom compared to the nucleus you can see just how inaccurate even this simple picture is.
Atomic dimensions
Hydrogen atomic radius: 3.7 x 10-11 m
Hydrogen nucleus radius: 8 x 10-16 m
You should appreciate that the nuclear radius is much smaller than the atomic radius by a factor of about 100,000. This means that the atom is mostly empty space with a very solid and tiny nucleus. This was originally demonstrated by the scattering experiments carried out by Ernest Rutherford.
Particle mass
The actual masses of these sub-atomic particles are very small and to the nearest whole number measured relative to the mass of a carbon-12 isotope being equal to 12 units:
Although these values suggest that the protons and neutrons are identical they do, in fact, have very slightly different masses, which is only of concern to us when considering changes in the structure in nuclear chemistry. Compared to the mass of the protons and neutrons the electrons have negligible mass and can be ignored when carrying out calculations involving mass.
Protons have a mass of 1 atomic mass unit. They are all together in the nucleus, but they cannot repel one another because of the strong nuclear force exerted by the protons and the neutrons. You could consider the neutrons to be the nuclear glue that holds the nucleus together.
|
A full description of the electron is rather complicated as they behave both as particles in some circumstances and waves in others. This has lead quantum physicists to calling them wavicles - things that possess the characteristics of both particles and waves.
|
|
This model allows us to use the atomic theory successfully to explain many observations in the microscopic world. Its use in modern particular science has been refined by the introduction of another model, which is rather more difficult to understand, called the quantum model.
Electrical charges
The forces that hold atoms together (and to one another) are largely electrical in nature (apart from the strong nuclear charge that binds the particles in the nucleus together).
Electrically charged particles have an associated electrostatic field, rather like a magnet has a magnetic field. If another electrical charge comes into this field it will feel a force of either attraction or repulsion, depending on whether the charge carried is opposite or the same.
 |
|
|
Like charges repel
|
Opposite charges attract
|
Overall, atoms are neutral, which means that they must have as many positive charges as negative charges.
Protons carry a single positive charge and the electrons carry a single negative charge, so in the neutral atom there are always the same number of protons and electrons.
The electrons are tiny in comparison to the protons and neutrons. The overall charge on an atom is zero, the charges of the electrons cancel out the positive charges of the protons in the nucleus.
Summary of fundamental particle charge and location
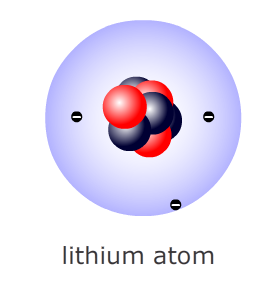
Example: A lithium atom has three protons in the nucleus along with four neutrons.
It has a total mass of 3 + 4 = 7 atomic mass units
It has 3 protons each with one positive charge so it must have three electrons each with one negative charge to cancel them out.
Therefore the atom has 3 protons, 4 neutrons and 3 electrons. Mass = 7.
The atomic mass number is represented by the symbol (letter) 'A'. This is not to be confused with the relative atomic mass Ar.
The mass number gives the integral number of nucleons, protons and neutrons found in the nucleus of an atom.
The relative mass is a value that is not necessarily integral that compares a mass to the mass of a carbon isotope, assigned a value of exactly 12.0000 units.
This is represented by the symbol (letter) 'Z'. It shows us the number of protons in an atom (and the number of electrons in a neutral atom.)
Example: How many protons and electrons does an atom of iron contain?
The atomic number of iron is 26 therefore it contains 26 protons
The number of electrons = number of protons, therefore there are 26 electrons
Any isotope of any element can be defined by using the A value, the Z value and the element symbol.
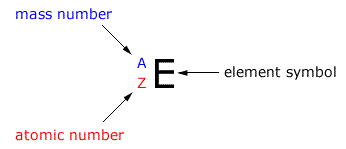
Using the values of A and Z it is possible to calculate the number of sub-atomic particles within any specific isotope of an element.
Example: Determine the number and type of sub-atomic particles in the following atom:

The atomic number is 1 therefore there is 1 proton and 1 electron
The mass number is 3 therefore there are (3-1) = 2 neutrons
Example: Determine the number and type of sub-atomic particles in the following atom:
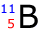
The atomic number is 5 therefore there are 5 protons and 5 electrons
The mass number is 11 therefore there are (11-5) = 6 neutrons
The system can be extended to cover ions simply by adding the charge onto the element symbol. It is important to remember that a positive ion has LOST electrons.
Example: Determine the number of electrons in the following ion:
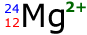
The atomic number is 12 therefore in a neutral atom there would be 12 protons and 12 electrons.
However, the charge is 2+ therefore the atom has LOST 2 electrons
The remaining electrons then = 10 electrons
Worked examples
Q112-01 40Ca, 39K, and 41Sc all have the same...- number of electrons.
- atomic number.
- mass number.
- number of neutrons.
|
The three atoms are all from different elements so their protons and electrons must be different. The mass number is stated in the question and is clearly different. Therefore they must all have the same number of neutrons.
Correct response = D |
Q112-02 If a neutral atom has an atomic number of 29 and a mass number of 61, then the atom must contain
- 90 neutrons
- 61 electrons
- 29 neutrons
- 29 electrons
|
The atomic number gives the number of protons. If the atom is neutral, the protons = the electrons. Therefore correct response = D |
Q112-03 Atom X has 9 protons, 9 electrons, and 10 neutrons. Atom Y has 10 protons, 10 electrons, and 9 neutrons. It can therefore be concluded that
- atom X and Y are isotopes.
- atom X is more massive than atom Y.
- atoms X and Y have the same mass number.
- atoms X and Y have the same atomic number.
|
The two atoms have different numbers of protons therefore they are from different elements and cannot be isotopes. They have different mass numbers and atom X is more massive than atom Y. Therefore correct response = B |
Q112-04 A proton has approximately the same mass as:
- a neutron
- an alpha particle
- a beta particle
- an electron
|
Protons and neutrons have approximately the same mass. Correct response = A |
Q112-05 What is the mass number of an atom which contains 28 protons, 28 electrons, and 34 neutrons?
- 28
- 56
- 62
- 90
|
Protons + neutrons = mass 28 + 34 = 62 |
Q112-06 Which atom contains exactly 15 protons?
- P-32
- S-32
- O-15
- N-15
|
The atomic number = the number of protons. Referring to the periodic table, atomic number 15 = phosphorus. Therefore correct response = A |
Q112-07 Which one of the following statements is not true about the atom 81Rb?
- It has mass number 35
- It contains 35 electrons
- It contains 46 neutrons
- Its nucleus contains 81 particles
|
Rubidium (Rb) has an atomic number of 35 therfore it has 35 protons and 35 electrons. The numberof neutrons is calculated by sbtracting the mass number from the number of protons so it has 81-35 = 46 neutrons. The number 81 represents the mass number and so response 'A' is incorrect. |
Q112-08 What is the mass number of an atom which contains 26 protons, 26 electrons, and 30 neutrons?
- 28
- 56
- 62
- 90
|
Mass number is given by number of protons plus number of electrons. Therefore mass number = 26 + 30 = 56 Therefore correct response = B |
Q112-09 Which of the following statements is INCORRECT?
- Protons are found in the nucleus of an atom
- Neutrons have no charge
- Electrons have a mass of 1 atomic mass unit
- The nucleus contains most of the atomic mass
|
Protons ARE found in the nucleus. Neutrons have no charge Electrons have almost no mass, therefore this is the incorrect statement. Therefore correct response = C |
Q112-10 Which of the following statements about numbers of sub-atomic particles is CORRECT?
- electrons plus neutrons equals protons
- protons minus neutrons equals atomic number
- neutrons plus protons equals mass number
- mass number minus electrons equals protons
|
Electrons are equal to protons (in a neutral atom) Protons minus neutrons will often give a negative value Nuetrons + protons = mass number - this is the correct statement Therefore correct response = C |
Q113-11 How many protons are
present in the atom: 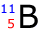
|
The number of protons is represented by the atomic number on the AZE system - in this case the red number 5. Therefore this boron atom has 5 protons |
Q113-12 How many electrons
are present in the atom: 
|
The number of electrons in a neutral atom (as opposed to an ion) is given by the atomic number on the AZE system - in this case the red number 13. Therefore this aluminium atom has 13 electrons |
Q113-13 How many neutrons
are present in the atom: 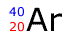
|
Argon has a mass number of 40 as shown by the blue number. The mass number is given as the sum of the protons and the neutrons. The protons are obtained from the red number 20, the atomic number. Therefore each argon atom contains 40-20 = 20 neutrons |
Q113-14 The atom  is used in medical tracer treatment of bone disorders (amongst other things). How many neutrons does each technetium atom contain?
is used in medical tracer treatment of bone disorders (amongst other things). How many neutrons does each technetium atom contain?
|
This technetium atom has an atomic number of 43 and a mass number of 99. It contains 99-43 = 56 neutrons |
Q113-15 An ion with 5 protons, 6 neutrons, and a charge of 3+ has an atomic number of:
- 5
- 6
- 8
- 11
|
Here the 3+ ion is merely a distraction. The atomic number is obtained from the number of protons, in this case 5. Therefore correct response = A |
Q113-16 One 40Ca2+ ion contains:
- 2 protons
- 18 electrons
- 21 neutrons
- 2 electrons
|
In a neutral atom the electrons = the protons = the atomic number, in this case 20. The calcium ion has lost two electrons, therefore number of electrons = 18 Correct response = B |
Q113-17 The symbol for a particular magnesium ion is 24Mg2+. The number of electrons contained is one of these ions is:
- 2
- 10
- 12
- 22
|
In the magnesium ion, two electrons have been lost (leaving an overall double positive charge). In a neutral atom, the electrons = the protons = the atomic number, in this case 12. Therefore the total number of electrons = 12 - 2 = 10 Correct response = C |
Q113-18 How many neutrons
are present in the ion: 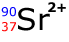
|
Ionic charge affects only the electrons in a particle. This strontium particle has a mass number of 90 and an atomic number (proton number) of 37. Therefore number of neutrons = 90 - 37 = 53 neutrons |
Q113-19 How many electrons
are present in the ion: 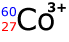
|
Cobalt has an atomic number of 27. In a neutral atom it has 27 electrons, however, in the Co3+ ion it has LOST three electrons. Therefore number of electrons left = 24 electrons. |
Q113-20 How many neutrons
are present in the atom: 
|
Potassium has a mass number of 39 as shown by the blue number. The mass number is equal to the sum of the protons and the neutrons. The protons are obtained from the red number 19, the atomic number. Therefore each potassium atom contains 39-19 = 20 neutrons |
| Now test yourself |
External video resources
Sub-atomic particles: Richard Thornley