Standard level
Atoms differ from one another suggesting that the arrangement of sub-atomic particles is different from atom to atom. One way that they differ is in terms of mass.
Syllabus ref: S1.2.2Structure 1.2.2 - Isotopes are atoms of the same element with different numbers of neutrons.
- Perform calculations involving non-integer relative atomic masses and abundance of isotopes from given data.
Guidance
- Differences in the physical properties of isotopes should be understood.
- Specific examples of isotopes need not be learned.
Tools and links
- Nature of science, Reactivity 3.4 - How can isotope tracers provide evidence for a reaction mechanism?
Isotope definition
Isotopes are atoms of a specific element that have a definite number of neutrons and consequently a different mass. In effect all atoms are isotopes of one element or another.
Most elements have several isotopes, some of which are stable, and others that spontaneously break apart releasing radioactivity.
For example, the element hydrogen has three isotopes, 1H, 2H and 3H. 1H is the most common of the isotopes and makes up 99.99% of any sample of hydrogen. 2H is also called deuterium and comprises the other 0.01% of naturally occurring hydrogen. The third isotope is called tritium and is not very common.
Tritium is radioactive and breaks apart spontaneously releasing radioactivity, in this case, a fast moving electron.
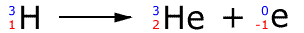
Note that the product of this radioactive process is helium. Effectively, one of the neutrons in the tritium nucleus has emitted an electron (called a beta particle) and turned into a proton.
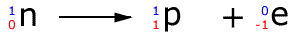
Summary of the hydrogen isotopes
Relative abundance
The 'relative abundance' of an isotope means the percentage of that particular isotope that occurs in nature. Most elements are made up of a mixture of isotopes. Clearly the sum of the percentages of the specific isotopes must add up to 100%.
Example:Chlorine has two isotopes 35Cl and 37Cl, with relative abundance of 75% and 25% respectively.
This means that in any naturally occurring sample of chlorine 75% of the atoms are Cl-35 atoms and 25% of the atoms are chlorine-37 atoms.
Properties of isotopes
Isotopes differ only in their number of neutrons. This means that they have identical electronic configurations and identical chemical properties. The masses of the isotopes affects any characteristic that depends on mobility or mass of the particles.
Density is defined as mass/volume therefore isotopes have different densities.
Diffusion is a process that is dependent on the density of the diffusing species. According to Graham's law, the rate of diffusion is proportional to the square root of the reciprocal density and hence the reciprocal relative mass (of a gas):

Example:How does the rate of diffusion of hydrogen differ from the rate of diffusion of deuterium?
- Hydrogen, H2, relative mass = 2
- Deuterium, D2, relative mass = 4
Applying Graham's law, relative rates of diffusion = 1/√2 : 1/√4
Therefore 1/1.414 : 1/2 = 0.71 to 0.5
Hydrogen diffuses 1.4 x faster than deuterium
This principle is put to use in the purification of uranium 235 for use in the atomic energy industry. The uranium occurs naturally as two isotopes 235U and 238U with relative abundances of approximately 0.28% to 99.71% with the remainder due to other isotopes.
Only the uranium 235 can be used for atomic energy and so needs to be concentrated in the sample. This is done by reacting the uranium with hydrogen fluoride to form uranium hexafluoride UF6, which is a volatile solid that can be converted to a gas at easily attainable temperatures.
- 235UF6
- 238UF6
Once in the gaseous form, use is made of the different diffusion rates of the two compounds. The relative masses of the two hexafluorides are so similar, the gases must be diffused using a series of centrifuges, each one increasing the percentage of the required uranium isotope in the mixture.
The uranium hexafluoride is then turned into uranium dioxide for use in fuel pellets.
Uses of isotopes
Isotopes are used in medicine, industry, and in many other applications. The danger of radioisotopes revolves around their ability to cause cell damage by ionising the atoms that are involved in molecules and hence, breaking bonds.
Radioisotopes may emit three different common types of radiation, alpha, beta and gamma radiation, depending on the specific atom.
- Alpha radiation consists of particles containing two protons and two neutrons (equivalent to a helium nucleus); is highly destructive to living tissue, but has very low penetration power and is stopped by a few centimetres of air. It is only seriously dangerous if ingested in some way.
- Beta radiation consists of highly energetic electrons. It has poorer ionising characteristics than alpha radiation, but has greater penetrating power.
- Gamma radiation is electromagnetic in nature and has the lowest ionising ability, but extremely great penetrating power.
14C - radiocarbon dating
Living organisms respire. Plants breathe in carbon dioxide and animals eat plants (and other animals!). The consequence is that all living things take up carbon throughout their lives.
The percentage of the isotope carbon 14 remains fairly constant in our atmosphere, as it is produced in the upper atmosphere by cosmic bombardment of naturally occurring carbon-12 in the form of carbon dioxide. At the same time the carbon 14 nuclei are decaying. There is an equilibrium between these two processes:
carbon-12 ⇋ carbon-14
This means that the proportion of carbon-14 compared to carbon-12 found in all living organisms is also constant. However, when a living organism dies it stops taking up both forms of carbon.
The carbon -14 isotope decays naturally with a half life of about 5,600 years. So, a simple procedure involving counting the radioemissions due to carbon-14 from a sample of material that was once alive, can be used to estimate how long ago it died.
Therapeutic applications
Cobalt-60 is used in hospitals as a beta emission source in the treatment of cancer
Beta rays are fast moving electrons. They can be focussed onto cancerous tissue to destroy it using a cobalt 60 source. This form of treatment is known as radiotherapy.
Iodine-131 and Iodine-125 are used as medical tracers and for treating certain cancers.
In several conditions the body can be scanned for problems using iodine, which is easily taken up by the body and transported through the lymphatic system. The isotopes 131I and 125I are easy to detect and short lived in the body.
Use is made of the destructive effect on cellular tissue to destroy cancer cells in treatment with radioisotopes. Radioactive sources are used that have a short lifetime in the body, but which can be focussed in their effects on tissues.
Technetium-99, for example is used in gammagraphy, a technique where a sample of the radioisotope is injected into the body. After a few hours the technetium circulates around the body and binds to areas of bone damage. By detection of areas of unusually high concentration of radiation, it is possible to identify bone injuries that do not show up on X-rays.
The bone scans of radiation emissions are called gammagrams.
Industrial applications
Detection of leaks in gas pipes by injection of a radioisotope into the pipeline and detecting where the radiation emerges. Beta emitters are used in measurement of thickness in the paper industry.
Nuclear energy generation
Both uranium-235 and plutonium-239 are neutron emitting radioactive isotopes. The neutrons emitted cause further events in neighbouring nuclei leading to chain reactions, which release large amounts of energy as the nuclei break apart (fission). This energy is used to heat up water into steam to drive turbines for electricity production.
Nuclear energy remains controvertial and there are strong arguments both for and against its use.
Other applications
Americium-241 is a man-made isotope used in smoke detectors.
Worked examples
Q114-01 A sample of gallium has a relative atomic mass of approximately 69.8. If the sample consists of two isotopes of masses 69.0 and 71.0 respectively, what is the approximate percentage of the lighter isotope in the sample?- 80.0 %
- 60.0 %
- 40.0 %
- 20.0 %
|
The relative mass is the weighted average of the isotopes, given by:
By inspection the relative mass lies closer to 69 than to 71, so there must be a greater percentage of the 69 isotope than the 71 isotope. There are two possible answers A and B. Carrying out the calculation for A [(80 x 69) + (20 x 71)]/100 = 69.4 which is incorrect Therefore the percentage of the lighter isotope = 60% (response B) This question can also be solved by assigning the percentage abundance of the lighter isotope as "x". The heavier isotope percentage abundance is then (100 - x). The mass of 100 atoms = 100 x relative atomic mass = 6980 and this equals 69x + 71(100 - x) = 7100 - 2x Hence 7100 - 2x = 6980 2x = 120 x = 60% (answer B) |
Q114-02 Separation of the isotopes of uranium requires a physical method rather than a chemical method because
- it is too dangerous to mix other chemicals with uranium.
- the isotopes are chemically the same element.
- the isotopes differ in number of neutrons.
- natural uranium contains only 0.7% U-235.
|
Isotopes have almost identical chemical properties and so must be separated by physical methods. Answer = response B |
Q114-03 There are two stable isotopes of carbon. They differ with respect to
- atomic mass
- number of protons
- atomic number
- electron configuration
|
Isotopes have different numbers of neutrons and therefore a different atomic mass Answer = response A |
Q114-04 The average atomic weight of elemental copper is reported as 63.5. Copper consists of two stable isotopes, 63Cu and 65Cu. Approximately what percent of naturally occurring copper is the 63Cu isotope?
- 30
- 50
- 70
- 90
|
The relative mass is the weighted average of the isotopes, given by:
By inspection the relative mass lies closer to 63 than to 65, so there must be a greater percentage of the 63 isotope than the 65 isotope. There are two possible answers C and D. Carrying out the calculation for C [(70 x 63) + (30 x 65)]/100 = 63.6 which is close Carrying out the calculation for D [(90 x 63) + (10 x 65)]/100 = 63.2 which is further away Therefore the the best answer = 70% (response C) |
Q114-05 Which symbols represent atoms that are isotopes?
- C-14 and N-14
- O-16 and O-18
- I-131 and Te-131
- Rn-222 and Ra-222
|
Isotopes must be the same element. Only response B fulfills this requirement. |
Q114-06 For an isotope of argon (Z = 18), the mass number is 40. The number of neutrons in this isotope is:
- 18
- 40
- 22
- the same as in any other isotope of argon
|
The number of neutrons is obtained by subtracting the atomic number from the mass number Therefore the number of neutrons = 40 -18 = 22 neutrons |
Q114-07 Boron has two isotopes, 10B and 11B, with relative abundances of 20% and 80% respectively. Which of the following is the relative atomic mass of boron?
- 10.5
- 11.0
- 10.8
- 10.2
|
The relative mass is the weighted average of the isotopes, given by:
Carrying out the calculation for boron [(10 x 20) + (11 x 80)]/100 = which is incorrect Therefore the relative atomic mass = 10.8 (response C) |
Q114-08 Which of the following is different for two isotopes of the same element?
- The atomic number
- The number of electrons
- The mass number
- The reactivity
|
There is a different number of neutrons in each isotope giving rise to a different mass number. |
Q114-09 Neon occurs as two isotopes of mass numbers 20 and 22. Its relative atomic mass is 20.2. What is the percentage of 20Ne in naturally occurring neon?
- 10
- 20
- 80
- 90
|
The relative mass is the weighted average of the isotopes, given by:
Carrying out the calculation for neon let the percentage of neon-20 = x, now the percentage of neon 22 = (100-x) [(20 x x) + (22 x (100-x))]/100 = 20.2 solving for x, 20x - 22x = 2020 - 2200 therefore x = 90 Therefore the percentage of neon 20 = 90% (response D) |
Q114-10 Hydrogen has three isotopes, protium with no neutrons, deuterium with one neutron and tritium with two neutrons. In a sample trapped in a meteorite, they are found to occur in the ratio 10: 1: 1. What is the relative atomic mass of the hydrogen in the sample?
- 1.10
- 1.25
- 1.50
- 2.00
|
In this case the relative mass is the weighted average of three different isotopes, but the process is the same. if the ratio is 10:1:1 then we can take the mass of 12 atoms and divide through by 12. for 'protium' 10 x 1 = 10 for 'deuterium' 1 x 2 = 2 for 'tritium' 1 x 3 = 3 total mass = 15 Average mass = 15/12 = 1.25 Therefore the relative atomic mass of hydrogen in the sample = 1.25 (response B) |
| Now test yourself |
External video resources
Physical properties of isotopes: Richard Thornley