Standard level
The world of microscopic particles does not behave in the same way as our everyday experience. The energy posessed by particles is quantised, i.e. can have only certain well defined levels. This concept is known as quantum theory.
Syllabus ref: S1.3.4Structure 1.3.4 - A more detailed model of the atom describes the division of the main energy level into s, p, d and f sub-levels of successively higher energies.
- Recognize the shape and orientation of an s atomic orbital and the three p atomic orbitals.
Guidance
Tools and links
- Structure 3.1 - What is the relationship between energy sub-levels and the block nature of the periodic table?

Electron arrangement
The electrons in atoms are arranged in energy levels. The lowest energy (most stable) energy level is the one closest to the nucleus. The first energy level can hold up to two electrons. Once it is full, the next energy level may then start to fill up.
However, each energy level itself is subdivided into subshells containing regions of space in which there is a 0.99 probability of finding electrons of a specific energy. These regions are called orbitals.
Atomic orbitals
The regions of space in which electrons are found were defined by Ernst Schröedinger in the early part of the 20th century, by solving equations relating their energy and the electrostatic forces to which they are subject. These regions of space are termed 'orbitals'
Electrons behave like particles in some respects and waves in others, but in all cases they behave as negative charges. They are very difficult to pin down in terms of location, however it is possible to define a region of space in which the probability of finding the negative charge is very close to certainty - about 99%. This region of space is called an orbital.
Each energy level is divided into sub-levels each of which in turn has a certain number of orbitals.
One atomic orbital can house up to two electrons, each with a different 'spin'. The word 'spin' is used to differentiate between the two different electrons. It does not imply that they actually spin, but studies show that they do have slightly different energies and the idea of spinning electrons allows us an easy image with which to differentiate between them. The first electron to enter an orbital spins up and the second spins down.
1st Energy level
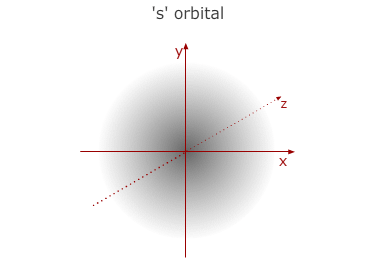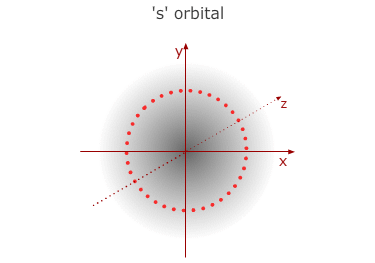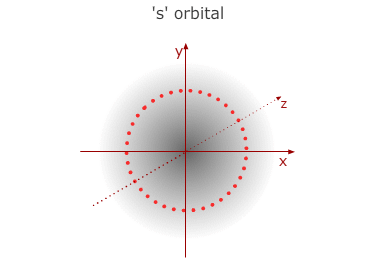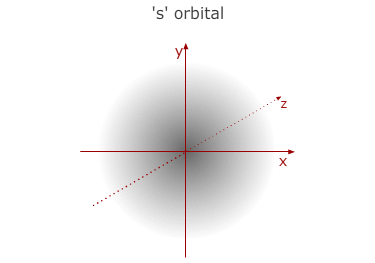 This is the first (lowest) energy level. It has no sub divisions
and consists of only one orbital with a spherical shape - called a 1s orbital.
(the '1' refers to the energy level).
This is the first (lowest) energy level. It has no sub divisions
and consists of only one orbital with a spherical shape - called a 1s orbital.
(the '1' refers to the energy level).
Like other orbitals, it can house a maximum of two electrons.
2nd Energy level
 This is the first energy level to be split into sub-levels, 's' and
'p'. The 's' sub-level once again has only one spherical orbital which is
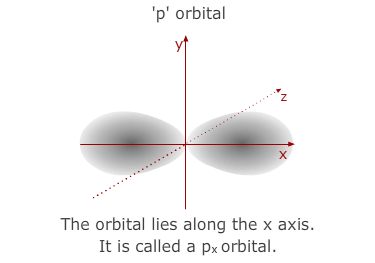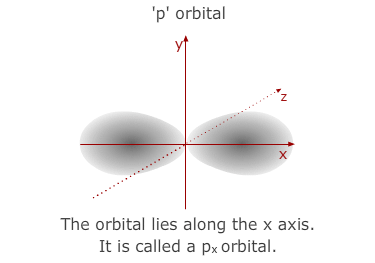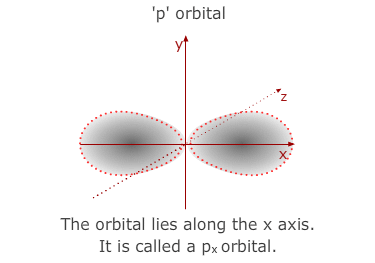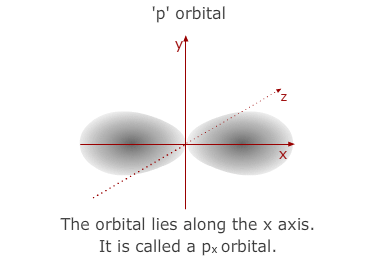
much larger than the 1s orbital from the 1st level. The 'p' sub-shell consists
of three atomic orbitals shaped a bit like double headed balloons. Each of
the orbitals is designated a letter x, y or z to indicate the axis that it
lies along.
This is the first energy level to be split into sub-levels, 's' and
'p'. The 's' sub-level once again has only one spherical orbital which is
much larger than the 1s orbital from the 1st level. The 'p' sub-shell consists
of three atomic orbitals shaped a bit like double headed balloons. Each of
the orbitals is designated a letter x, y or z to indicate the axis that it
lies along.
The 'p' orbitals are of slightly higher energy than the 's' orbital, consequently the 's' orbital fills up before the 'p' orbitals become occupied.
3rd energy level
The third energy level is split into three sub-levels, 's', 'p' and 'd' sub-shells. Each of these has corresponding atomic orbitals.
- The 3s sub-shell consists of one 3s orbital, spherical in shape, like the 1s and 2s orbitals, but much larger.
- The 3p sub-shell contains 3 separate 'p' orbitals, the same shapes and orientations as the 2'p' orbitals but, once again, larger in volume.
- The 'd' sub-shell consists of five 'd' orbitals with specific shapes.
The energies of the orbitals is in the order s<p<d
4th energy level
Contains 's', 'p', 'd', and 'f' orbitals (7 of them), with relative energies s<p<d<f
Question Where do the letters used to represent the types of orbitals come from?
The letters used to represent the orbitals actually come from the lines produced on spectra by electrons making transitions involving these subshells. Historically, the 's' stands for 'sharp', the 'p' stands for principal', the 'd' for diffuse and the 'f' for 'fundamental'
Summary
The energy levels are split into sub-levels, each with orbitals that have shapes corresponding to the type of sub-shell.
|
Energy level
|
sub-shells
|
type
|
orbitals
|
|
1
|
1
|
s
|
s
|
|
2
|
2
|
s,p
|
s, px,py,pz
|
|
3
|
3
|
s,p,d
|
s,px,py,pz,dxy,dxz,dyz,d(x2-y2),
dz2
|
|
4
|
4
|
s,p,d,f
|
as above plus 7 'f' orbitals
|
External video resources
The Shapes of 's' and 'p' orbitals: Richard Thornley
