Standard level
The word "Aufbau" comes from the German meaning "construction". It is how the electrons fill up the available atomic orbitals in order of increasing energy, according to certain rules.
Syllabus ref: S1.3.5Structure 1.3.5 - Each orbital has a defined energy state for a given electron configuration and chemical environment, and can hold two electrons of opposite spin.
- Sublevels contain a fixed number of orbitals, regions of space where there is a high probability of finding an electron.
- Apply the Aufbau principle, Hund’s rule and the Pauli exclusion principle to deduce electron configurations for atoms and ions up to Z = 36.
Guidance
- Full electron configurations and condensed electron configurations using the noble gas core should be covered
- Orbital diagrams, i.e. arrow-in-box diagrams, should be used to represent the filling and relative energy of orbitals.
- The electron configurations of Cr and Cu as exceptions should be covered.
Tools and links

Atomic orbitals
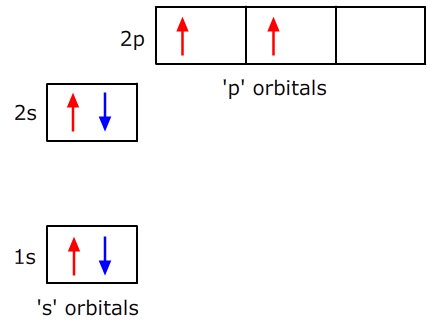
Each energy level is split into sub-levels (except energy level 1). The sub-levels in turn, contain orbitals that can hold a maximum of two electrons per orbital.
- Level 1 contains only 1 's' orbital
- Level 2 contains 1 's' and three 'p' orbitals
- Level 3 contains 1 s, three p and five 'd' orbitals
- Level 4 contains one 's', three 'p', five 'd' and seven 'f' orbitals
As stated above the electrons fill up the orbitals in order of increasing energy from the lowest energy orbitals upwards. This process is subject to certain 'rules
Pauli's exclusion principle
This states that no two electrons can be identical within an atom. Simply
stated, it means that only two electrons can fit into each atomic orbital
and they must have opposite spins. By convention, we say that one electron
spins up,  and the other down
and the other down  represented by up and down arrows.
represented by up and down arrows.
Hund's rule
This states that electrons entering orbitals that have the same energy (degenerate orbitals, represented by boxes on the same level) must be filled by parallel electrons (unpaired electrons), before the electrons become paired up.
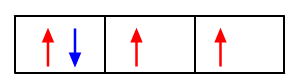
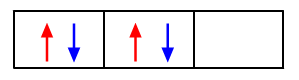
Example: In the electronic configuration of carbon 1s2 2s2 2p2, the electrons fill up in the following way:
Anomalous configurations
The Aufbau principle works fairly well for the first 38 elements, but after that it starts to break down. Even so, there are two configurations that do not seem to fit into the pattern. These are chromium and copper.
Chromium (24 electrons) has an expected configuration of [Ar] 4s2 3d4. However, the actual configuration is [Ar] 4s1 3d5.
This is explained by suggesting that there is some energetic advantage to the atom to have a half-full set of 'd' orbitals, and that this is enough to cause one of the 4s orbital electrons to occupy the last orbital in the 3d series.
Copper (29 electrons) also has an anomalous configuration with the expected [Ar] 4s2 3d9 giving way to [Ar] 4s1 3d10.
Once again, this is explained by the extra stability due to a full set of 3d orbitals, providing the incentive for the 4s electron to be housed in the last 3d orbital.
Example: Which of the following atoms has/have one or more unpaired electrons?
- I. Iron
- II. Copper
- III. Zinc
To answer the question it is necessary to look at the electronic configuration of each atom.
- Iron [Ar] 4s2 3d6
- Copper [Ar] 4s1 3d10
- Zinc [Ar] 4s2 3d10
Iron has 6 'd' electrons to fit into 5 'd' orbitals. As the first five must enter with parallel spin it has 4 unpaired electrons
Copper has a single unpaired electron in the 4s orbital and all the '3d' orbitals are full
Zinc has a full set of 4s and 3d orbitals
Therefore, of the three elements, only iron and copper have unpaired electrons.
Electronic configuration of ions
Ions are formed from atoms by the addition or removal of electrons depending on whether the atom is a metal or a non-metal.
Metals lose electrons forming positive ions. The number of electrons lost depends on the metal atoms. Group 1, 2 and 3 elements lose 1, 2 and 3 electrons respectively to give a noble gas configuration.
The 'd' block (transition) metals have variable oxidation states and may lose a variable number of electrons. The first electrons lost by the first row 'd' block metals are the 4s electrons. After these have been removed the '3d' electrons are successively removed until the required ion is obtained.
Example: Show the electronic configuration of iron(II) and iron(III) ions.
iron(II) has a charge of 2+ and has consequently lost 2 electrons. Iron (III) has a charge of 3+ and has lost 3 electrons.
Iron (at no. 26) has an electronic configuration = 1s2 2s2 2p6 3s2 3p6 4s2 3d6
iron(II) has a configuration of 1s2 2s2 2p6 3s2 3p6 4s0 3d6
iron(III) has a configuration of 1s2 2s2 2p6 3s2 3p6 4s0 3d5
Worked examples
Q135-01 Which statement is correct about electron orbitals and energy levels?- Yttrium, Y, (Z = 39) is the first element in the periodic table with an electron in a f sub-level.
- The maximum number of electrons in one d orbital is 10.
- The maximum number of electrons in the 4th main energy level is 18.
- In a main energy level, the sub-level with the highest energy is labelled f.
|
A is incorrect as the 'f' orbitals start in the fourth energy level after lanthanum B is incorrect as an orbital can hold a maximum of two electrons regardless of its type C is incorrect as the fourth level holds s, p, d and f orbitals making 1 + 3 + 5 + 7 orbitals each with two electrons = 32 D is correct as the highest sub-level is named the 'f' level. |
Q135-02 Which is correct about the element tin (Sn) (Z = 50)?
|
Number of main energy levels containing electrons
|
Number of electrons in highest main energy level
|
|
|
A
|
4
|
4
|
|
B
|
4
|
14
|
|
C
|
5
|
4
|
|
D
|
5
|
14
|
|
Tin lies in period 5 of the periodic table and group 4 in the 'p' block. This means that it has electrons in the 5s and 5p orbitals which contain a total of four electrons. Response C |
Q135-03 How many electrons are there in all the d orbitals in an atom of xenon?
- 10
- 18
- 20
- 36
|
Xenon appears in the periodic table at the end of the 5th energy level. It has a full 3d and 4d sub shell, so it's 'd' orbitals contain 20 electrons |
Q135-04 1s2 2s2 2p6 3s2 3p6 3d3 4s2 is the ground state electronic configuration of which of the following?
- Ca
- Sc
- Zn
- V
|
The electronic configuation is that of Vanadium [Ar] 4s2 3d3 |
Q135-05 The electron configuration for Mn2+ is:
- [Ar] 4s2 3d3
- [Ar] 3d5
- [Ar] 4s1 3d5
- [Ar] 4s1 3d4
|
The ground state electronic configuration of manganese is [Ar] 4s2 3d5 Transition metal ions are formed by loss of the 4s electrons and so the electronic configuration of the 2+ ion is [Ar] 3d5 |
Q135-06 Which statement about the electron configuration of electrons in the Cs atom is correct?
- The outermost two electrons are paired in the same atomic orbital.
- The 4f shell is completely full.
- Only one of the 55 electrons is involved in most interactions of Cs with other atoms.
- The 4f shell is only partially filled.
|
Caesium is in goup 1 of the periodic table. Its outer shell contains only one 's' electron. This electron is respnsible for bonding; the correct response is C |
Q135-07 Which atom has the correct ground state electron configuration?
- Cl: [Ne] 3s1 3p6
- Mn: [Ar] 4s1 3d5
- Cu: [Ar] 4s2 3d8
- As: [Ar] 4s2 4d10 4p3
|
Chlorine has a configuration [Ne]3s2 3p5 Manganese has a configuraiton: [Ar] 4s2 3d5 Copper has an anomalous configuration of [Ar] 4s1 3d10 The correct answer is Arsenic with a configuration of [Ar] 4s2 4d10 4p3 |
Q135-08 The correct number of unpaired electrons in the ground state of a neutral cobalt atom is:
- 1
- 2
- 3
- 4
|
The ground state electronic configuration of cobalt is [Ar] 4s2 3d7 The seven 'd' electrons fit into five 'd' orbitals by first entering with parallel spin into all five orbitals and then pairing up in the first two. This leaves 3 unpaired electrons |
Q135-09 Which electron configuration describes a neutral atom in an excited state?
- [Xe] 6s2 4f14 5d10 6p3
- [He] 2s1
- [Ne] 3s1 3p1
- [Ar] 4s1 3d5
|
We are looking for a configuration in which the electrons do not fulfil the Aufbau principle and have at least one electron in an orbital of higher energy. At first sight either C or D seems to fit this requirement. However, the configuraiton of D is the anomalous configuration of chromium and therefore IS a ground state. The correct answer is [Ne] 3s1 3p1 - response C |
Q135-10 A certain element has the electronic configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3. Which oxidation states would this element most likely show?
- +2 only
- +3 only
- +2 and +5 only
- +2, +3, +4, +5
|
Inspection of the configuration reveals that the outermost electrons are 4s2 3d3. This is a 'd' block transition element and hence has a variable oxidation state. It first forms an 2+ ion by loss of the 4s electrons There are still three 'd' electrons that can be lost, allowing it to develop a III, IV or V oxidation state Correct response - D |
| Now test yourself |
External video resources
Electronic configurations: Richard Thornley
