Standard level
Atoms of all kinds (except the noble gases) are inherently chemically unstable on their own. We know this because they tend to exist in conjuction with other atoms, either in the form of elements, or compounds.
Ionic bonding takes place between metals and non-metals creating a giant structure made up of ions of opposite charges, packed together in a giant crystal lattice. Ionic bonding is the theory used to explain how these giant lattices can be formed from their constituent atoms.
In this section we take a look at the various processes involved.
Syllabus ref: S2.1.1Structure 2.1.1 - When metal atoms lose electrons, they form positive ions called cations. When non-metal atoms gain electrons, they form negative ions called anions.
- Predict the charge of an ion from the electron configuration of the atom.
Guidance
- The formation of ions with different charges from a transition element should be included.
Tools and links
- Structure 3.1 - How does the position of an element in the periodic table relate to the charge of its ion(s)?
- AHL Structure 1.3 - How does the trend in successive ionization energies of transition elements explain their variable oxidation states?
Electronic configuration and energy
The noble gases have full outer shells and are stable as individual atoms. All of the other atoms have incomplete outer shells and are chemically reactive. The inference is that a full outer shell confers stability on an atomic structure.

Metal atoms have very few electrons in their outer shells and can gain stability by losing these electrons. The electrons cannot just be ejected into space as their removal actually requires energy.
However, if the electrons are transferred to a non-metal atom, forming an ionic pair, then the resultant lowering of energy caused by the mutual attraction of the ions is more than enough to compensate for the energy required to ionise the metal.
Electron transfer
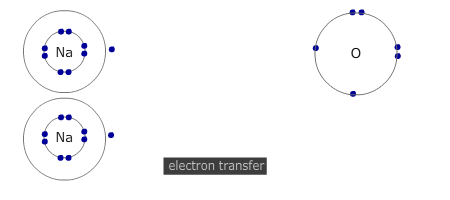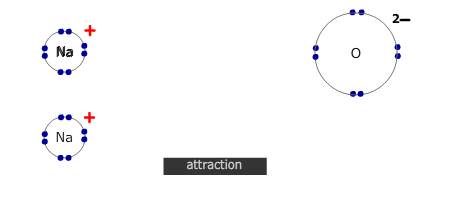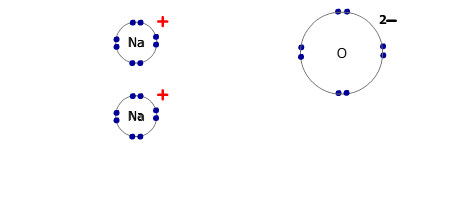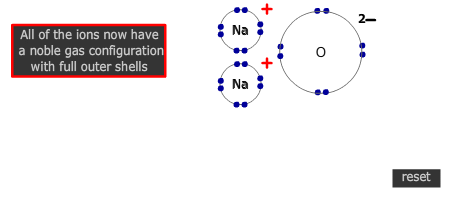
Electrons are transferred from metal atom to non-metal atom. In the course of the process both the metal and non-metal atoms attain a noble gas configuration.
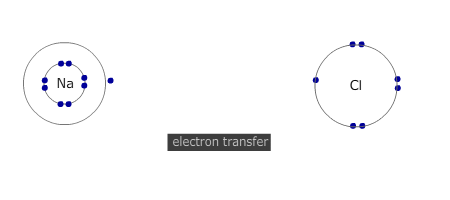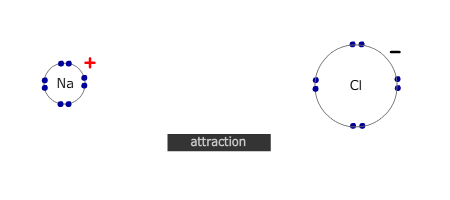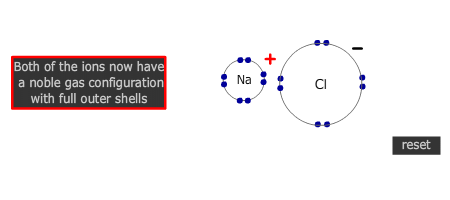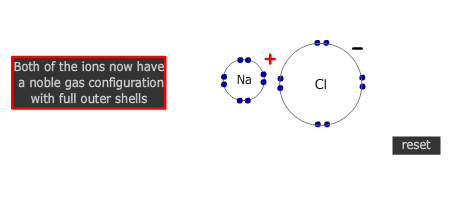
The number of atoms that react together is determined by the number of electrons that each atom needs to lose, or gain, to attain a full outer shell. Group 2 metals need to lose 2 electrons. Both of their outer (valence) shell electrons MUST be transferred to suitable non-metal atoms. If the non-metal can only accept one electron, then two metal atoms are needed (and vice versa).
Negative and positive ions
The electrical charge on an ion depends on the number of electrons gained or lost by an atom. This, in turn, depends on the group number of the atom in the periodic table.
Metals are found on the left hand side of the periodic table, they always form positive ions. The magnitude of the positive charge is the same as the group number of the metal.
- Group 1 metals form +1 charge ions, eg Li+, Na+ and K+
- Group 2 metals form +2 charge ions, eg Mg2+, Ca2+ and Ba2+
Non-metals are from the right hand side of the periodic table, they have nearly full outer shells and form negative ions by capturing electrons. The magnitude of the negative charge on the ion is the same as the number of electrons needed to attain a full outer shell.
- Group 16 elements require 2 more electrons to attain a full outer shell, the ionic charge is 2-, eg O2- and S2-.
- Group 17 elements require 1 more electron to attain a full outer shell, the ionic charge is 1-, eg F- and Cl-.
Remember that ALL compounds are neutral so the positive charges of the ions MUST be balanced by the negative charges of the ions.
Example: What is the formula of the compound formed between the ions Mg2+ and N3-?
The electronic charges must cancel out. Clearly 2+ cannot cancel out 3- so we need another 2+. This makes 4+ altogether which now cannot be cancelled out by the 3-.
So we need another 3- making 6- altogether.
Now if we add another 2+ we get 6+ to cancel out the 6-.
Therefore the formula comes from Mg2+Mg2+Mg2+N3-N3- = Mg3N2
Notice that the ionic charges are NOT included in the formula.
The transition metals form positive ions, but the charge on the ion can't be predicted from the periodic table. In fact transition metals have a variable oxidation state, meaning that they form several ions. For this reason the valency (which is the same as the ionic charge) has to be included in the compound name, so that we know the type of ion involved.
Examples: Names of transition metal compounds
copper(II) sulfate, cobalt(II) chloride
Normally transition metals form 2+ and 3+ ions, although there are exceptions, such as Cu+, Ag+.
Naming simple ions
The positive ions do not change their names from that of the metals. For example an ion formed from a copper atom is simply called a copper ion. However, the ions formed from non-metals change the end of the name in the ionic form. Simple ions change the ending to -ide.
Examples:
- Chlorine → chloride
- Bromine → bromide
- Oxygen → oxide
- Nitrogen → nitride
Ionic bonding - summary
Elements that are able to form positive ions transfer electrons to elements that are able to form negative ions. In general, this means reactions between metals and non-metals, but be aware that there are exceptions. When the electronegativity difference between the metallic element and the non-metallic element is small, covalent compounds can form. BeCl2, AlCl3 are both covalent compounds
The process follows these steps:
- Transfer of electrons
- Formation of positive and negative ions with full outer shells
- Electrostatic attraction of the positive and negative ions, forming a giant ionic lattice.
Worked examples
Q261-01 What happens when sodium and oxygen combine together?- Each sodium atom gains one electron
- Each sodium atom loses one electron
- Each oxygen atom gains one electron
- Each oxygen atom loses one electron
|
Sodium is in group 1 and so must lose 1 electron to attain a full outer shell. Oxygen is in group 16 and needs to gain two electrons. The only correct choice is that each sodium atom loses one electron. |
Q261-02 What is the formula of an ionic compound formed by element X (group 2) and element Y (group 16)
- X3Y
- X2Y
- XY2
- XY
|
Group 2 elements need to lose two electrons and group 16 elements need to gain two electrons. There can be direct transfer of two electrons from one atom of X to one atom of Y. Therefore the formula is XY |
Q261-03 Element X (group 2) and element Y (group 17). Which ions will be present in the compound formed when X and Y react together?
- X+ and Y-
- X2+ and Y-
- X+ and Y2-
- X2+ and Y-
|
Group 2 elements form 2+ charged ions and group 17 elements form 1- charged ions. X2+ and Y- |
Q261-04 Which statement gives incorrect information about the formation of NaCl(s) from Na(s) and Cl2(g)?
- Na is oxidized and Cl reduced.
- An ionic lattice forms.
- Both the ionization of Na and electron attachment to Cl are exothermic processes.
- When NaCl forms from its gaseous ions, energy is released.
|
By definition oxidation is the removal of electrons and reduction is the addition of electrons. (OILRIG) When sodium reacts with chlorine the sodium loses an electron to the chlorine atom, hence sodium gets oxidised and chlorine gets reduced. NaCl is ionic so an ionic lattice forms. Ionization of sodium is not an exothermic process, therefore this is the incorrect response. |
Q261-05 The compound formed between magnesium and oxygen is primarily:
- Ionic with a formula MgO
- Ionic with a formula MgO2
- Covalent with a formula MgO
- Covalent with a formula MgO
|
Magnesium (group 2) is a metal and oxygen (group 16) is a non-metal. The difference in electronegativity is large, therefore the type of compound formed is ionic and has the formula MgO. |
Q261-06 Explain why aluminium fluoride conducts electricity in the liquid state, but not in the solid state.
Answer
|
Aluminium is from group 13 and fluorine is from group 17. Fluorine is the most electronegative atom and always forms ionic bonds with metals. Molten aluminium fluoride has ions that are free to move and carry a current. In solid form, however, the ions are in fixed positions and cannot carry current. |
Q261-07 Magnesium chloride and silicon chloride have very different properties. Give the formula and physical states of each chloride at room temperature.
Answer
|
Magnesium chloride is an ionic substance (although with a small degree of covalent character), whereas silicon chloride is totally covalent. Their formulas are MgCl2 and SiCl4, physical states at room temperature are solid and liquid respectively. |
Q261-08 The letters W, X, Y and Z represent four consecutive elements in the periodic table. The number of electrons in the highest occupied energy levels are: W:3, X:4, Y:5, Z:6
Write the formula for an ionic compound formed from W and Y showing the charges
Answer
|
Element W has three electrons in the outer (valence) shell. Element Y has 5 electrons in the outer (valence) shell. Both have valencies of three and form a compound with formula WY. |
Q261-09 The oxides of magnesium and silicon have high melting points whereas the oxides of phosphorus (P4O6) and sulfur (SO2) have low melting points. Explain the difference in melting points by referring to bonding and structure in each case.
Answer
|
Magnesium oxide is giant ionic. Silicon dioxide is giant covalent, large amounts of energy are required to break the bonds holding these giant structrures together. However, both phosphorus(III) oxide and sulfur(IV) oxide are simple covalent substances, in which the molecules are held together by weak forces of attraction. In the case of sulfur(IV) oxide these forces are due to dipole - dipole interactions and in the case of phosphorus(III) oxide, the forces are due to dispersion attractions. Simple molecular structures require far less energy to overcome the weak forces holding the structure together and they melt at much lower temperatures. |
Q261-10 State the bonding in the oxides of sodium, magnesium, silicon and phosphorus.
Answer
|
Sodium oxide and magnesium oxide are giant ionic structures. Silicon dioxide is a giant covalent structure and phosphorus oxide is a simple covalent structure. |
External video resources
Deduce the ions from groups 15, 16 , 17 gain electrons: Richard Thornley
Deduce the ions formed by groups 1, 2 ,13: Richard Thornley
Describe how ions can be formed by electron transfer: Richard Thornley
