Standard level
Ionic bonding takes place between metals and non-metals creating a giant structure of repeating ions. In this section we take a look at the nature of the ionic bond.
Syllabus ref: S2.1.2Structure 2.1.2 - The ionic bond is formed by electrostatic attractions between oppositely charged ions.
- Deduce the formula and name of an ionic compound from its component ions, including polyatomic ions.
- Binary ionic compounds are named with the cation first, followed by the anion. The anion adopts the suffix “ide”.
- Interconvert names and formulas of binary ionic compounds.
Guidance
- The following polyatomic ions should be known by name and formula: ammonium NH4+, hydroxide OH–, nitrate NO3–, hydrogencarbonate HCO3–, carbonate CO32–, sulfate SO42–, phosphate PO43–.
Tools and links
- Reactivity 3.2 - Why is the formation of an ionic compound from its elements a redox reaction?
- AHL Structure 2.2 - How is formal charge used to predict the preferred structure of sulfate?
- AHL Reactivity 3.1 - Polyatomic anions are conjugate bases of common acids. What is the relationship between their stability and the conjugate acid’s dissociation constant, Ka?
Ionic or covalent?
An ionic bond is an electrostatic force between positive and negative ions. This electrostatic force in non-directional, it acts in all directions. The strength of the force depends on the magnitude of the charges on the ions and the sum of the ionic radii.

But what determines whether a compound is ionic or covalent?
If the bonding atoms have a large difference in electronegativity then this causes transfer of electrons and the formation of ionic compounds. As the difference in electronegativity decreases, the bond develops covalent character until eventually it becomes essentially covalent.
Notice that this process is not black and white. The bond type changes gradually from pure ionic to pure covalent, passing through all degrees.
Pure ionic >>> ionic with covalent character >>> polarised covalent >>> pure covalent
Pure ionic compounds are formed by group 1 metals when combining with non-metals. These are highly electropositive, having electronegativity values of between 0.7 and 1.0.
However, when the electronegativity value of the metal is higher, the bonding has a degree of covalent character. This means that the negative ion electron density is distorted and drawn towards the metal ion. There is some electron density between the two particles, typical of covalent bonding. This can be seen on electron density maps produced in X-ray crystallography. Instead of the electron density being symmetrical around the ions, it is distorted towards the positive ions.
Aluminium chloride - a case history
.gif) The
classic example is aluminium chloride. Aluminium is a metal from group 13
and consequently forms Al3+ ions. However, it is not very electropositive
and the high charge density of the small Al3+ ion allows it to
polarise the negative charge cloud on negative ions formed from atoms of lesser
electronegativity.
The
classic example is aluminium chloride. Aluminium is a metal from group 13
and consequently forms Al3+ ions. However, it is not very electropositive
and the high charge density of the small Al3+ ion allows it to
polarise the negative charge cloud on negative ions formed from atoms of lesser
electronegativity.
Aluminium oxide is an ionic compound, but aluminium chloride is only ionic in the solid state at low temperatures. At higher temperatures it becomes covalent. This is because the high charge density Al3+ ion can polarise the Cl- charge cloud, making an ionic bond with a high degree of covalent character, so much so that AlCl3 is usually considered to be covalent.
The difference in electronegativity between aluminium (1.5) and chlorine (3.0) is 1.5 units. This could be taken as a rough guide for the limit between ionic and covalent bonding.
When metals bond to non-metals if the difference in electronegativity is greater than 1.5, then the compound would be expected to be ionic, less that 1.5 and covalency is expected. It should be stressed that this is only an approximation and it is easy to find exceptions.
Polyatomic ions
Single ions are stable due to the completed outer shell. Groups of atoms, covalently bonded together, can also produce stable structures by addition, or removal of electrons, to create groups of atoms in which all of the atoms have full outer shells and the whole structure carries an electrical charge. Such arrangements are called polyatomic ions.
Polyatomic ions behave very much like simple (monoatomic ions), in that they form compounds, which are giant structures of repeating ionic units (lattices), with metal ions or other polyatomic, oppositely charged ions.
You must be familiar with several of these ions, their formulas, charges and structures
Common polyatomic negative ions (anions)
Students should be familiar with the following polyatomic ions: ammonium, hydroxide, nitrate, hydrogencarbonate, carbonate, sulfate and phosphate.
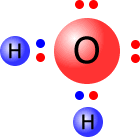
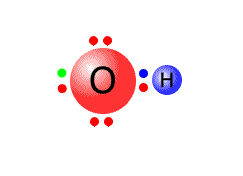
The simplest of the polyatomic ions is the hydroxide ion. This can be thought of as a water molecule that has lost a hydrogen ion (proton).
The sulfate ion comes from sulfuric acid reacting with bases and active metals. It has four pairs of electrons coordinated from the central sulfur atom to the four oxygen atoms. In some text books a structure is shown that suggests octet expansion, however this is simply not necessary.
 |
Counting up the electrons in the valence shell of the four oxygen atoms and the sulfur we get 5 x 6 = 30. To obtain the structure of the sulfate ion with all outer shells full we need 32 electrons. Thus, the combined atoms have gained two electrons and the ion has a double negative charge. |
|
the sulfate ion SO42-
|
Nitrate ions are formed by reactions of nitric acid with bases and metals.
 |
Total electrons around the valence shells in the nitrate ion = 24. The sum of valence electrons from the constituent atoms = 5 (from nitrogen) + 3 x 6 (from oxygen) = 23. Thus the ion has gained one electron with respect to its constituent atoms, therefore it must carry a negative charge. Note: All three N-O bonds are the same length and the ion is a totally symmetrical trigonal plane (bond angle 120º. |
|
the nitrate ion NO3-
|
Carbonate ions, CO32-, are the anions formed from carbonic acid (a solution of carbon dioxide in water).
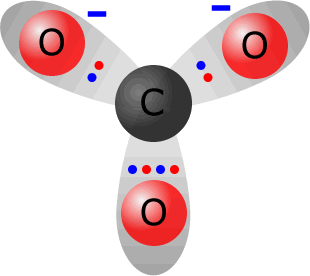 |
Total electrons around the valence shells in the carbonate ion = 24. The sum of valence electrons from the constituent atoms = 4 (from carbon) + 3 x 6 (from oxygen) = 22. Thus the ion has gained two electrons with respect to its constituent atoms, therefore it must carry a double negative charge. Note: All three C-O bonds are the same length and the ion is a totally symmetrical trigonal plane (bond angle 120º. |
|
the carbonate ion CO32-
|
Hydrogencarbonate ions, HCO3-, are formed from carbonate ions by coordinating a hydrogen ion onto one of the oxygen atoms. Sodium hydrogencarbonate, for example, is formed by partial neutralisation of sodium carbonate solution by an acid.
Na2CO3(aq) + HCl(aq) → NaHCO3(aq) + NaCl(aq)
Only group 1 metal hydrogencarbonates are able to exist as solid compounds. The hydrogen carbonates of group 2 metals can be prepared in solution, but decompose when the water evaporates:
Ca(HCO3)2(aq) → CaCO3(s) + CO2(g) + H2O(l)
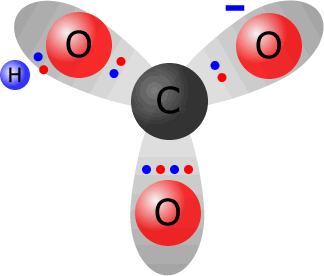 |
Total electrons around the valence shells in the hydrogen carbonate ion = 24. The sum of valence electrons from the constituent atoms = 4 (from carbon) + 3 x 6 (from oxygen) + 1 (from hydrogen) = 23. Thus the ion has gained one electron with respect to its constituent atoms, therefore it must carry a single negative charge. |
|
the hydrogencarbonate ion HCO3-
|
There are many other negative ions encountered in chemistry, but they all conform to the concept of stability due to full outer shells of electrons. The ionic charge can be calculated by examining the difference in electron numbers between the Lewis structure of the ion with full outer shells and the sum of the valence electrons of the constituent atoms.
Polyatomic positive ions (cations)
Stable positive polyatomc ions are not too common, the most common is the ammonium ion. Ammonia is a base with a lone pair of electrons on the nitrogen atom.
.gif)
.gif)
The lone pair acts as a base and can capture a hydrogen ion to form the ammonium ion according to the equation:
NH3 + H+ → NH4+
The ammonium ion behaves in many ways like a group 1 cation. It forms a whole series of salts, which are all white, soluble and stable at room temperature. However, many of the salts decompose on heating back into ammonia and another molecule.
Example: Action of heat on ammonium chloride:
NH4Cl → NH3 + HCl
Other positive ions found in chemistry include NO+, NO2+, PH4+, CH3+. The only ones stable enough to form compounds are those with complete valence shells around all of the constituent atoms. This is the case for PH4+, but not for CH3+.
Worked examples
Q262-01 Which compound has the greatest ionic character- MgS
- HCl
- CO2
- CaO
|
The greatest ionic character is show by the pair of elements with the largest electronegativity difference. In this case, CaO. |
Q262-02 Which compound is most likely to be ionic?
- GaAs
- ScBr3
- NO2
- ClO2
|
The greatest eletronegativity difference is seen in the compound ScBr3. Only scandium and gallium are metals, and gallium is a only weakly metallic in group 13. |
Q262-03 Which statements are generally true about the melting points of substances?
- I - Melting points are higher for compounds containing ions than for compounds containing molecules.
- II - A compound with a low melting point is less volatile than a compound with a higher melting point.
- III - The melting point of a compound is decreases by the presence of impurities.
- I only
- I and III only
- II and III only
- I, II and III
Correct response I and III only |
Q262-04 What kind of ions have, in general, the strongest interaction with water molecules in aqueous solution?
- ions with large electric charge and larger diameter
- ions with small electric charge and small diameter
- ions with large electric charge and small diameter
- ions with small electric charge and large diameter
|
Charge density is given by the charge/size ratio. This is the largest when the ion has a large electric charge and a small diameter. |
Q262-05 Tin(II) chloride is a solid with a melting point of 246 °C; tin(IV) chloride is a liquid with a freezing point of - 33 °C. These properties can be explained by
- the greater covalence of SnCl4 compared with that of SnCl2.
- the greater formula weight of SnCl4 compared with that of SnCl2.
- the greater degree of ionic character of SnCl4 compared with that of SnCl2.
- the greater number of ionic and molecular particles in SnCl4 with SnCl2.
|
The tendency towards covalency lowers the melting point. The facts can be explained by tin(IV) chloride being covalent whereas tin(II) chloride is ionic. Hence option A. |
Q262-06 Which compound has the least covalent character?
- SiO2
- Na2O
- MgCl2
- CsF
|
Least covalent is the same as saying most ionic. The greatest difference in electronegativity is between caesium and fluorine, hence CsF. |
Q262-07 Which compound dissolved in water to form a solution that does not conduct electricity?
- HCl
- NaCl
- CH3CH2OH
- CH3COOH
|
Only simple covalent substances that do not dissociate in solution are non-conductors. The only covalent substance in the list is CH3CH2OH |
Q262-08 Which is the most volatile substance?
- Chlorine
- Fluorine
- Sodium chloride
- Sodium fluoride
|
Volatile means that it turns into a gas easily. This is true for simple molecular substances such as chlorine and fluorine. The element with the lowest boiling point is the one with the weakest intermolecular forces, in this case dispersion forces. This is fluorine, due to its lower relative mass. |
Q262-09 Which statement is true for most ionic compounds?
- They contain elements of similar electronegativity?
- They conduct electricity in the solid state
- They are coloured
- They have high melting and boiling points
|
Ionic substances are giant structures with many strong forces of electrostatic attraction and have high melting and boiling points |
Q262-10 Aluminium and oxygen combine together to form a compound that has a high melting point and is a good conductor of electricity when molten. Write an equation to represent the formation of this compound and explain in terms of structure and bonding why it has these properties.
Answer
|
Aluminium and oxygen react to form aluminium oxide. All metal oxides are ionic, as the difference in electronegativity is large. Hence, aluminium oxide consists of a giant ionic lattice of aluminium ions, Al3+ and oxide ions O2-. 4Al + 3O2 → 2Al2O3 Giant ionic lattices are held together by electrostatic forces. As the charge on the ions increases so do these forces. In aluminium oxide the aluminium ions have a 3+ charge and the oxide ions have a 2- charge. This makes the lattice very strong and high temperatures are required to melt it. However, once the ionic lattice is melted (becomes molten), the ions separate and become free to move. Al2O3(s) → 2Al3+(l) + 3O2-(l) These free ions can then carry electrical current. |
