Standard level
Covalent bonding usually occurs between non-metal atoms. It is the simplest kind of chemical bonding.
Syllabus ref: S2.2.1Structure 2.2.1 - A covalent bond is formed by the electrostatic attraction between a shared pair of electrons and the positively charged nuclei.
- The octet rule refers to the tendency of atoms to gain a valence shell with a total of 8 electrons. Deduce the Lewis formula of molecules and ions for up to four electron pairs on each atom.
Guidance
- Lewis formulas (also known as electron dot or Lewis structures) show all the valence electrons (bonding and non-bonding pairs) in a covalently bonded species.
- Electron pairs in a Lewis formula can be shown as dots, crosses or dashes.
- Molecules containing atoms with fewer than an octet of electrons should be covered.
- Organic and inorganic examples should be used.
Tools and links
- Nature of science - What are some of the limitations of the octet rule?
- Structure 1.3 - Why do noble gases form covalent bonds less readily than other elements?
- Structure 2.1 - Why do ionic bonds only form between different elements while covalent bonds can form between atoms of the same element?
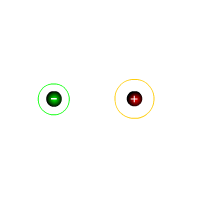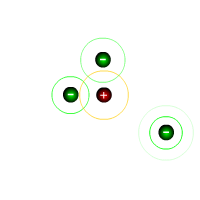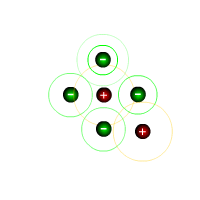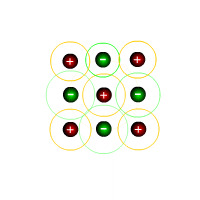
The covalent bond
Atoms are held together in covalent bonding by means of shared pairs of electrons. This constitutes a single covalent bond.
The various theories as to how and why these bonds are formed are discussed below. It is important to recognise that formation of covalent bonds (or any other type of bond) is an exothermic process, one that releases energy. Similarly, to break a bond always requires energy.
Any cleavage (breakage) of covalent bonds involves a chemical reaction and new substances are necessarily formed.
Bonding theory
Atoms are unstable unless they have fully occupied outer shells of electrons. We know from observation and inference that atoms bond together to make larger structures.
In order to explain how this happens, different theories are proposed that explain these observations. All of these theories revolve around the accepted idea that opposite charges attract (electrostatic attraction).
The scientific method
A theory helps to explain all of the observable facts. If it fits all of the evidence, then it is adopted as a useful explanation of how the universe works. However, if further observations throw up anomalies, or observations that cannot be explained by the current theory, then it must be either adapted, or even discarded completely.
Linear combination of atomic orbitals
This theory of bonding proposes that molecules are formed by overlapping regions of space, allowing atoms to mutually share pairs of electrons. This pair of electrons is then held in a position between the two nuclei of the atoms holding them together. We can consider this theory by looking at the hydrogen molecule.
The hydrogen molecule
The hydrogen molecule is the simplest structure formed between atoms. In this case two hydrogen atoms share one pair of electrons between them, forming a diatomic molecule.
The negative charge clouds (atomic orbitals) overlap, placing a region of negative charge between the two hydrogen nuclei.
The electrons in each atom is also attracted to the nucleus of the neighbouring atom. The atoms are pulled together. This view of chemical bonding is known as covalent bonding as the valence electrons of both the hydrogen atoms are shared. ('co' = shared and 'valent' = valence electrons)
The regions of space in which electrons are found are called orbitals and we say that this bond arises from direct overlap of atomic orbitals. This is sometimes called the Linear Combination of Atomic Orbital Theory (LCAO).
This model works very well for a simple picture of bonding between atoms. The nuclei of the atoms are held to one another by the positive - negative - positive attractions caused by electron pairs between the nuclei.
Full outer energy shells
The only atoms that occur in nature not combined into some kind of structure are the inert gases. These all have full outer (valence) shells. It seems to be that this particular electronic configuration is stable for some reason.
| Atom | configuration |
|---|---|
| helium | 2 |
| neon | 2,8 |
| argon | 2,8,8 |
| krypton | 2,8,18,8 |
| xenon | 2,8,18,18,8 |
Covalent bonding occurs between atoms so that they can attain a full outer shell by SHARING electrons.
In the case of hydrogen molecules, above, the atom would have a full outer shell if it were to attain two electrons. By an arrangement in which two atoms share one pair of electrons, each atom achieves the requirement. The system is now stable, it does not change further.
Covalent bonding usually occurs between non-metal atoms; they attain a full outer shell of electrons by sharing electrons. However, as we shall see in the following section there are exceptions.
Otherwise known as Lewis structures, dot-cross representations show the electrons in the outer shell (valence electrons) of all of the atoms involved in a molecule. Covalent bonds consist of electron pairs and there are also lone, or non-bonding pairs.
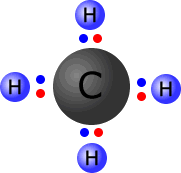
Conventionally, either dots or crosses can be used to represent the electrons in each outer shell:
In the image at the left, the four hydrogen atoms are bonded to the central carbon atom by means of covalent bonds. Each bond consists of a pair of electrons, conveniently coloured to distinguish those electrons that 'originally' came from carbon from those that 'belong' to the hydrogen atoms.
Atoms (with the exception of the noble gases) are not stable on their own. They MUST bond to produces structures in which the electronic arrangement is stable. In 99% of cases this means that the outer shell has a full 'octet', or eight electrons.
Lewis structures
The number of outer shell electrons may be found from the position of the
atom in the periodic table. For instance, fluorine is in group 17 and so
it has seven outer (valence) electrons.  All
single covalent bonds are represented by a pair of electrons, each bonding
atom providing one for the pair. There are two fluorine atoms in the fluorine
molecule and so there are 15 electrons altogether. Two electrons are used
in the single F-F bond leaving 12 electrons to share over the two atoms. These
are arranged as three non-bonding pairs per atom.
All
single covalent bonds are represented by a pair of electrons, each bonding
atom providing one for the pair. There are two fluorine atoms in the fluorine
molecule and so there are 15 electrons altogether. Two electrons are used
in the single F-F bond leaving 12 electrons to share over the two atoms. These
are arranged as three non-bonding pairs per atom.
Example: Draw the Lewis structure of the ammonia molecule.
Ammonia has the formula NH3, therefore there is one N atom and three H atoms.
- Nitrogen is in group 5, hence it has 5 electrons in the outer shell
- Hydrogen has one outer electron only; 3 x hydrogen = 3 electrons
Total outer electrons = 3 + 5 = 8
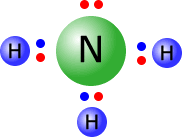 Each
N-H bond requires 2 electrons, therefore 3 x N-H = 6 electrons used
in the three bonding pairs. Remaining electrons = 8 - 6 = 2 electrons,
therefore one non-bonding pair.
Each
N-H bond requires 2 electrons, therefore 3 x N-H = 6 electrons used
in the three bonding pairs. Remaining electrons = 8 - 6 = 2 electrons,
therefore one non-bonding pair.
Double and triple bonds
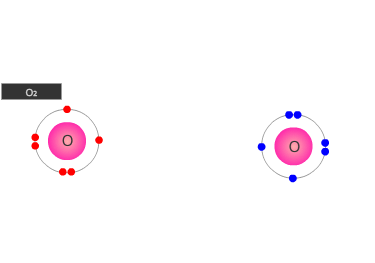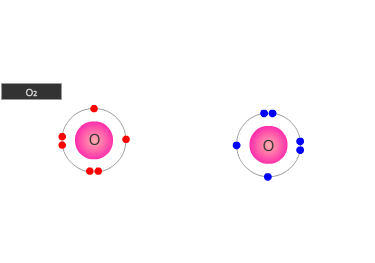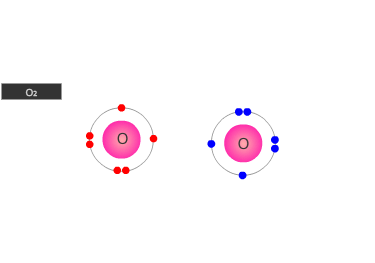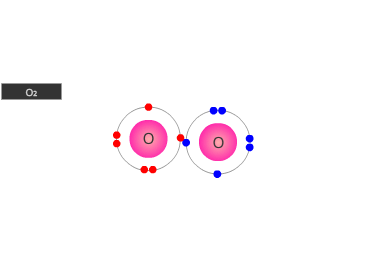
A double bond is shown as two shared pairs of electrons, each of the bonded
atoms provide two electrons for the bond. In the oxygen molecule at the left,
their are two shared pairs of electrons giving a stable octet (eight) of electrons
around each oxygen atom. 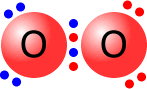
In triple bonds there are three pairs of electrons holding the two atoms
together. The molecule shown at the right is the ethyne (acetylene) molecule.
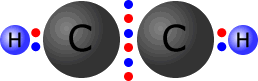 Each
of the carbon atoms (group IV) originally started with 4 outer electrons.
In the ethyne molecule at the right, both of the carbon atoms now have a full
octet (eight) of electrons and the two hydrogen atoms have a full first shell,
with two electrons. There are no non-bonding pairs.
Each
of the carbon atoms (group IV) originally started with 4 outer electrons.
In the ethyne molecule at the right, both of the carbon atoms now have a full
octet (eight) of electrons and the two hydrogen atoms have a full first shell,
with two electrons. There are no non-bonding pairs.
Electron deficient molecules
These are strucures in which the outer (valence) shells do not have a full
octet of electrons. This may be because the molecule has been formed from
electropositive atoms that have few electrons in the outer shell, or possibly
the molecule is just an exception. Such molecules are by definition unstable
and there are few exceptions that need to concern us here.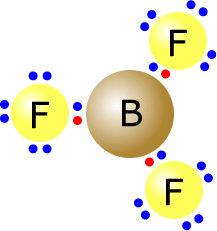
Boron trifluoride is electron deficient, as the boron atom is relatively electropositive and only has three outer electrons. The fluorine atoms themselves do have a full outer shell, but the boron atom in the centre has only six electrons in its valence shell.
Boron trifluoride Lewis Structure
Another example of electron deficiency is the beryllium dichloride molecule, BeCl2.
To construct the Lewis structure of beryllium dichloride we have to first
consider the number of outer electrons in the central atom Beryllium is from
group II and has two electrons in the outer shell. It bonds to two chlorine
atoms. 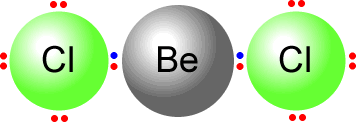 Chlorine
can only use a single bond when bonding as it has 7 electrons in its outer
shell. There are two chlorine atoms therefore the beryllium shares two pairs
of electrons, one with each chlorine. However this then leaves beryllium with
only four electrons in its outer (valence) shell. It is electron deficient.
Chlorine
can only use a single bond when bonding as it has 7 electrons in its outer
shell. There are two chlorine atoms therefore the beryllium shares two pairs
of electrons, one with each chlorine. However this then leaves beryllium with
only four electrons in its outer (valence) shell. It is electron deficient.
Odd electron species
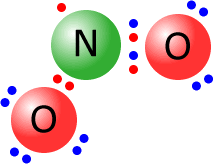 Occasionally molecules may manage to become stable even though they do not have a full
outer shell. This is not common, but is the case for nitrogen(IV) oxide, NO2,
at elevated temperatures. As you can see from the Lewis structure, there are
only seven electrons around the central nitrogen.
Occasionally molecules may manage to become stable even though they do not have a full
outer shell. This is not common, but is the case for nitrogen(IV) oxide, NO2,
at elevated temperatures. As you can see from the Lewis structure, there are
only seven electrons around the central nitrogen.
It is impossible to draw a Lewis structure in which all of the atoms are surrounded by an octet of electrons. However, the molecule does exist. It is reactive and at lowish temperatures soon pairs up to form a more stable structure, N2O4, in which all of the atoms do have a full octet.
2NO2⇋ N2O4
Summary of covalent bonding
- Covalent bonding happens when non-metal atoms combine
- Electron pairs are shared to give a full outer shell of valence electrons to each atom
- The final units are molecules of atoms joined together by shared pairs of electrons
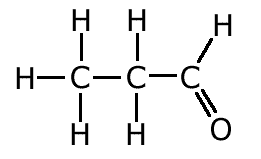
The covalent bond (shared pair) may be represented by a line to show typical stick structures such as:
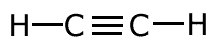 |
 |
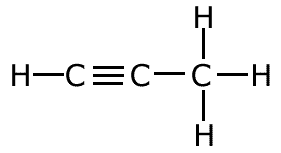 |
|
ethyne
|
propanal
|
propyne
|
A single line is drawn to represent one pair of electrons. A double bond containing two pairs of electrons between atoms is shown as a double line, and three pairs, a triple bond, as a three lines. It is not possible for two atoms to share more than three pairs of electrons.