Higher-level only
Molecular orbital theory is a more sophisticated and holistic view of covalent bonding that builds upon the previous theories of linear combination of atomic orbitals in conjuction with delocalisation, resonance and Hybridization.
Syllabus ref: S2.2.15Structure 2.2.15 - Sigma bonds σ form by the head-on combination of atomic orbitals where the electron density is concentrated along the bond axis. (HL)
- Pi bonds π form by the lateral combination of p-orbitals where the electron density is concentrated on opposite sides of the bond axis.
- Deduce the presence of sigma bonds and pi bonds in molecules and ions.
- Include both organic and inorganic examples.
Guidance
Tools and links
Molecular orbitals
Atoms and molecules are the physical manifestations of the stable structures that exist in our universe. An atom is an arrangement of charged particles which, except for the noble gases, cannot exist on its own for very long.
Atoms join up to form either metallic structures, molecules or ionic structures. Effectively, a molecule is just an arrangement of charged particles which is particulary stable. It is essentially a multi-centre noble gas type atom.
In the same way that atoms have orbitals in which to house their electrons, so do molecules. The shape and orientation of the atomic orbitals, as discovered by Ernst Schroedinger, was based on the energies and interactions between the positive and negative particles.
It is a perfectly logical assumption that molecules have orbitals of their own, with distinct shapes and orientations depending on the multiple nuclear centres and their interaction with the electronic charges.
These molecular orbitals are constructed according to certain rules.
- 1 The sum of the atomic orbitals of the constituent atoms must equal the sum of the molecular orbitals.
- 2 The sum of the energies of the molecular or orbitals must equal the sum of the energies of the atomic orbitals
- 3 Only occupied orbitals contribute to the energy sum.
- 4 Orbitals between nuclear centres contribute to bonding (bonding orbitals).
- 5 Orbitals not between nuclear centres do not contribute to bonding (anti-bonding orbitals).
- 6 For every bonding orbital there must be a corresponding anti-bonding orbital.
Sigma bonds
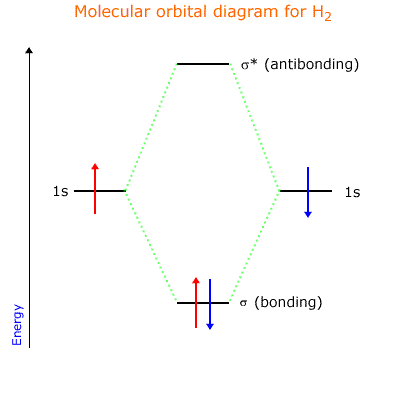
A sigma bond is a molecular orbital formed by the direct orbital overlap along an axis of two appropriately shaped orbitals, for example 's' and 'p' orbitals, or 's' and 's' orbitals.
The result is a region of electron density (electron domain) that resides between two nuclear centres. There is also a sigma antibonding orbital formed (rule 1 above) that does not contribute to bonding. This can be shown using the simplest example, the hydrogen molecule.
These are molecular orbitals that can be thought of as being formed by lateral overlap of parallel orbitals (usually 'p') resulting in regions of electron density above and below the axis that joins the two nuclear centres.
The molecular orbital model defines a region of space above and below the bond axis in which there are a pair of electrons with parallel spins. These electrons exert an electrostatic attraction on the two nuclei that helps to bond them together. This can be appreciated by examining the bond enthalpy of the carbon-carbon single and double bonds.
- Carbon - carbon single (sigma) bond energy = 346 kJ mol-1
- Carbon - carbon double (sigma + pi) bond energy = 602 kJ mol-1
This suggests that the pi component has a bond energy of 602 - 346 = 256 kJ mol-1.
Hence, the pi component is weaker than the sigma component, as would be expected by the relative distance from the nuclei of the electrons in the two bonds.
The existance of a pi molecular orbital is accompanied by a pi* antibonding orbital, in which any electrons do not contribute to bonding.