Higher-level only
Hybridization is a model that is used to fit the shape and orientation of atomic orbitals to the empirical evidence that provide us with the shapes of molecules. It is NOT a process.
Syllabus ref: S2.2.16Structure 2.2.16 - Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals for bonding. (HL)
- Analyse the hybridization and bond formation in molecules and ions.
- Identify the relationships between Lewis formulas, electron domains, molecular geometry and type of hybridization.
- Predict the geometry around an atom from its hybridization, and vice versa.
Guidance
- Include both organic and inorganic examples.
- Only sp, sp2 and sp3 hybridization need to be covered.
Tools and links
Hybridization
This is the idea that atoms involved in bonding rearrange their orbitals shapes and energies in order to produce orbitals that can overlap successfully with suitable orbitals on adjacent atoms and be used in bonding.
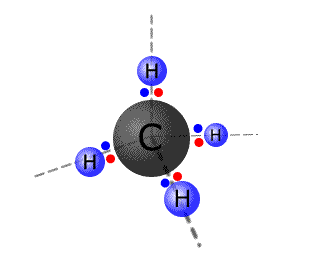
Carbon, for example, forms four bonds with hydrogen to make the molecule CH4. Studies show that the methane molecule is symmetrical and the hydrogen atoms are arranged in a tetrahedral fashion around the central carbon atom.
 However,
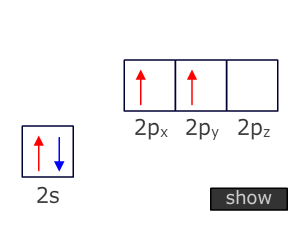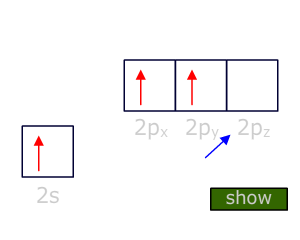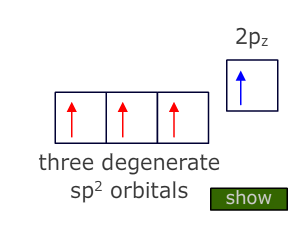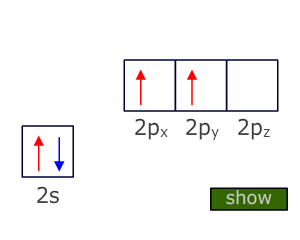
the atomic orbitals in the outer shell of carbon have two electrons in a 2s
orbital and 2 electrons in two 2p orbitals. There are three problems here:
However,
the atomic orbitals in the outer shell of carbon have two electrons in a 2s
orbital and 2 electrons in two 2p orbitals. There are three problems here:
- The 2s orbital is spherical and can't overlap with adjacent atoms.
- The 2s orbital is full. It can't share an electron from another overlapping orbital.
- There are only two singly occupied orbitals available for overlap and shaing electrons suggesting that carbon should only form two bonds and that these bonds would be at 90º to one another.
Carbon atoms overcome these problems by reforming the orbitals to give four degenerate orbitals, each of which is singly occupied. This is called Hybridization.
It must be stressed that Hybridization is simply an model to explain the difference between the shapes and orientation of the orbitals found in atoms and molecules. It gives us a process that explains how one situation can change into another.
Hybridization in double and triple bonds
Double bonds
Hybridization can also explain the formation and shape of molecules that contain double and triple bonds.
Using carbon as the example, in the case of a double bond the central atom needs to only bond to three other atoms. To do this it needs three degenerate orbitals which it obtains by Hybridization of the three valence orbitals that have the lowest energy, i.e. the 2s and two of the 2p orbitals.
Triple bonds
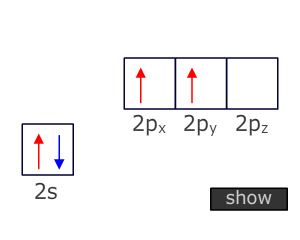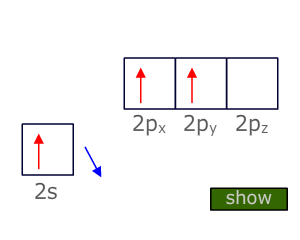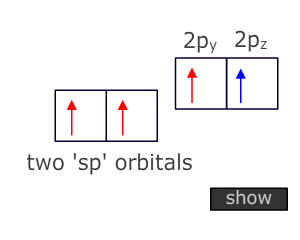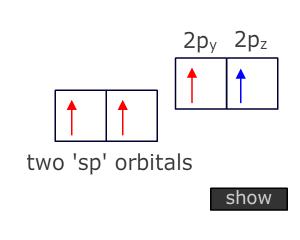
Atoms that are triple bonded can only bond to two other atoms. This means that two degenerate orbitals are needed. These are provided by Hybridization of the two orbitals from the valence shell that have the lowest energy, i.e. the 2s and onle of the 2p orbitals.
This Hybridization scheme produces two 'sp' orbitals at 180º to one another (linear) that can be used for direct orbital overlap (sigma bonding) and leaves two unchanged 'p' orbitals that can be used for lateral overlap with suitable orbitals on adjacent atoms (pi bonding).
It must be stressed that Hybridization occurs in all molecules, not just carbon-containing molecules. It is a simple matter to spot sp3, sp2 and sp Hybridization by the number and type of bonds on an atom.
Carbon, for example, forms four bonds. If all four bonds are single then the Hybridization is sp3. If there is one double bond then it is using sp2 Hybridization and if there is a triple bond then the Hybridization is sp.

Nitrogen forms only three bonds with other atoms and in nitrogen containing compounds one double bond is indicative of sp2 Hybridization, just like in carbon. In the ammonia molecule the four electron pairs are arranged in a tetrahedral orientation using sp3 Hybridization.
In the hydrogen cyanide molecule the nitrogen is attached to the carbon atom by a triple bond. Both atoms are sp hybridised.
In summary:
- If an atom has only single bonds then it is using sp3 Hybridization.
- if an atom is attached with one double bond then it is sp2 hybridised
- If an atom is attached by a triple bond then it is sp hybridised.
Worked examples
Q226-01 Appropriate Hybridization schemes for the C atoms in molecular CH3CO2H are:- sp3 and sp
- sp3 and sp2
- sp2 and sp
- sp3 and sp3
The correct response is sp3 and sp2 |
Q226-02 sp3 Hybridization would not be appropriate for the central atom in:
- SiF4
- [PCl4]+
- XeF4
- [Me4N]+
|
The molecule XeF4 has six pairs of electrons around the central atom and cannot involve sp3 Hybridization. |
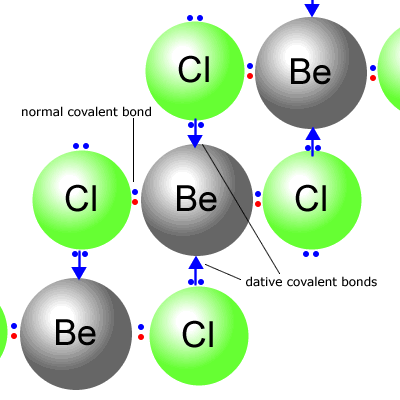
Q226-03 Suitable Hybridization schemes for Be in BeCl2(g) and BeCl2(s) are, respectively:
- sp and sp
- sp3 and sp3
- sp3 and sp
- sp and sp3
|
BeCl2(g) is simple molecular and has four electrons around the central atom in two degenerate orbitals. The lowest energy orbitals in the second energy level are an 's' and a 'p' orbital. Therefore BeCl2(g) used sp Hybridization. In the solid form the BeCl2 has electrons donated from chlorines of adjacent molecules into the empty orbitals on the beryllium atom making a giant lattice structure. Each beryllium atom is now associated with four other atoms, it therefore uses sp3 Hybridization. The correct answer is sp and sp3
|
Q226-04 Which of the following molecules contain a central atom which is sp2 hybridised?
- H2SO4
- H2CO3
- ICl2
- H3CCH3
|
sp2 Hybridization requires an atom to have only three regions of electron charge. The only structure that fulfills this requirement is the carbon atom in H2CO3. |
Q226-05 Which molecule utilizes sp3 Hybridization according to Valence Bond Theory?
- NH3
- BF3
- BeF2
- XeF4
|
For sp3 Hybridization there must be four regions of electric charge around the atom. Only NH3 has four regions, three bonding pairs and one lone pair. |
Q226-06 The structure for dimethyformamide is shown below: How many C and N atoms are sp3, sp2, and sp hybridised?
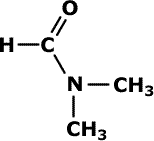
- 4 (sp3), 0 (sp2), 0 (sp)
- 3 (sp3), 0 (sp2), 1 (sp)
- 3 (sp3), 1 (sp2), 0 (sp)
- 2 (sp3), 2 (sp2), 0 (sp)
|
The carbonyl carbon is sp2 hybridised and the lone pair on the nitrogen atom of the amide laterally overlaps with the carbonyl 'pi' system, becoming sp2 as well. The other carbon are sp3 hybridised. Correct response D (sp3), 2 (sp2), 0 (sp) |
Q226-07 What is the Hybridization of nitrogen atoms I,II, III and IV in the following molecules?
| H2NINIIH2 | HNIIINIVH |
| I | II | III | IV | |
| A. | sp2 | sp2 | sp3 | sp3 |
| B. | sp3 | sp3 | sp2 | sp2 |
| C. | sp2 | sp2 | sp | sp |
| D. | sp3 | sp3 | sp | sp |
|
The first nitrogen has four regions of electrical charge (three bonds and a lone pair), the second nitrogen has the same. Nitrogen number III has only three regions of electrical charge, the same as nitrogen IV. The correct response is sp3, sp3, sp2, sp2. |
Q226-08 Identify the types of Hybridization show by the carbon atoms in the molecule CH2CH2CH2COOH
- I sp
- II sp2
- III sp3
- I and II only
- I and III only
- II and III only
- I, II and III
|
The first two carbon atoms are bonded to only three other atoms and have no lone pairs. They employ sp2 Hybridization. The third carbon atom is sp3 hybridised and the last carbon atom in the COOH group is sp2 hybridised. Correct response II and III only |
Q226-09 Which types of Hybridization show by the carbon atoms in the molecule CH2=CH-CH3
- I sp
- II sp2
- III sp3
- I and II only
- I and III only
- II and III only
- I, II and III
|
The first two carbon atoms are bonded to only three other atoms and have no lone pairs. They employ sp2 Hybridization. The remaining carbon atom is sp3 hybridised. Correct response II and III only |
Q226-10 NO3- is trigonal planar and NH3 is trigonal pyramidal. What is the approximate Hybridization of N in each of these species?
| N in NO3- | N in NH3 | |
| A. | sp2 | sp3 |
| B. | sp2 | sp2 |
| C. | sp3 | sp2 |
| D. | sp3 | sp3 |
|
The NO3- ion is trigonal planar meaning that it must use sp2 Hybridization, whereas the trigonal pyramidal NH3 must involve sp3 Hybridization. Correct response sp2 and sp3 |