Standard level
Students must be aware of the importance of metals in society and the need to make and use alloys.
Syllabus ref: 2.4.3Structure 2.4.3 - Alloys are mixtures of a metal and other metals or non-metals. They have enhanced properties.
- Explain the properties of alloys in terms of non-directional bonding.
Guidance
- Illustrate with common examples such as bronze, brass and stainless steel. Specific examples of alloys do not have to be learned.
Tools and links
- Structure 1.1 - Why are alloys more correctly described as mixtures rather than as compounds?
The nature of alloys
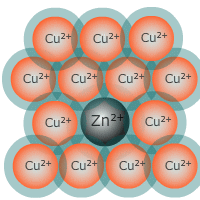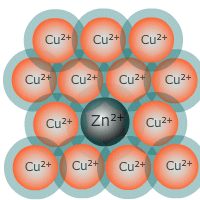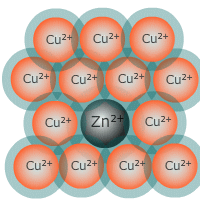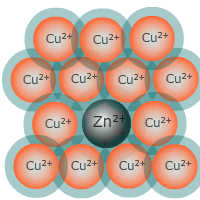
An alloy is a mixture of metals, although occasionally may include non-metals. Due to the nature of metallic bonding and the close packed structures they adopt, it is easy to slip other metal atoms into the lattice that modify the properties of a metal to suit different purposes.
For example, copper is a relatively soft metal, which used to be used for coinage. However, it very rapidly lost the prints on the surface of the coins and they became smooth and relatively difficult to use. The solution was to use an alloy of copper and nickel, which is much harder wearing. Nowadays, the majority of coinage is made from an alloy of these two metals.
Undesirable properties of metals may be softness, easy corrosion, poor conductivity etc. These can often be overcome by the addition of a suitable alloying metal.
Bronze
This alloy of copper and tin was so important in its day that a whole age was named after it. The approximate ratio of copper to tin is 85:15, but many other elements may also be added.

Metal packing
The way in which the atoms arrange themselves together in a metallic structure is known as the packing. A simple model treats the packing as if the particles were small spheres. The most efficient way in which the particles can pack together (least empty space) is known as "close packing".
In close packed structures 74% of all of the available volume is taken up leaving 26% for the "gaps" in the structure.
There are two forms of close packed structure, hexagonal close packed and cubic close packed. The difference between the two lies in the position of every third layer.
Close packing in metal structures
Relationship between structure and property in alloys
Metals are typically malleable, ductile, shiny conductive materials, with relatively high melting points.
Addition of impurities in the form of other metals or even non-metals can attentuate these properties and provide the new substance (alloy) with properties more suited to the design requirements.
When all of the atoms are the same in a metal, the particles are able to slide more easily over each other. This makes pure metals more malleable.
Alloy strength
Alloys can be made stronger than the original metal by addition of other elements whose incorporation into the structure prevents the particles sliding over one another easily.
Hence, metals can be hardened to be more durable.
Coinage metals, such as copper and silver have been largely superceded by alloys such as cupro-nickel, that can be manufatured to give percentage compositions with the desirable colour and hardness.
Alloy resistence to chemical attack
Alloys can be made more resistent to chemical attack by addition of other elements whose incorporation into the structure prevents oxidation.
A good example is the use of stainless steels rather than iron.
Modification of melting point
For purposes such as welding it is useful to manufacture alloys that melt at much lower temperatures than the original metal.
Solder is a generic terms for alloys usually of lead and tin used in plumbing and electronic components. Other elements such as indium and silver may also be added.
Modification of electrical conductivity
Metals are always conductors of electricity, but some are better than others. The delocalised electron bands that hold metal structures together also allow for conduction.
The more electrons in this delocalisation band, the better the conductivity. In general, alloys have lower conductivity than pure metals.
One such use of lowering conductance and increasing resistance is in the use of nickel-chromium alloys and iron-chromium-aluminium alloys in commercial heating elements.
Iron and steel
The most commonly used metal in society is steel. Steel is made by blowing oxygen through the molten iron manufactured by the Bessemer (blast furnace) process to remove excess carbon by oxidation to carbon dioxide.
Steel is iron with some carbon impurity and usually other metals are added to adopt the properties of the steel to that desired. Manganese may be added to steel used for springs, tungsten for greater hardness, molybdenum for ball bearings, etc. etc..