Standard level
The 20th century has been described as the age of plastic. From the serendipidous discovery of polythene in 1898 by the German Chemist Hans von Pechmann to the equally fortunate accidental discovery of an industrial process for synthesising poly(ethene) in 1933 by the English ICI (Imperial Chemical Industry) scientists Fawcett and Gibson, synthetic polymers have become both ubiquitous and an environmental concern in everyday life.
Syllabus ref: S2.4.4Structure 2.4.4 - Polymers are large molecules, or macromolecules, made from repeating sub-units called monomers.
- Describe the common properties of plastics in terms of their structure.
Guidance
- Examples of natural and synthetic polymers should be discussed.
Tools and links
- Structure 3.2 - What are the structural features of some plastics that make them biodegradable?
What is a polymer?
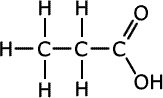
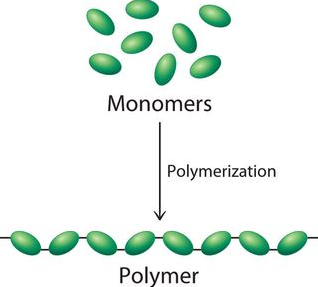
Polymers are large molecules composed of repeated sub-units, known as monomers, which are bonded together to form long chains. These materials are ubiquitous in our everyday lives, manifesting in various forms such as plastics, rubber, and DNA. The versatility of polymers stems from their unique molecular structure, which can be engineered to exhibit a wide range of physical and chemical properties.
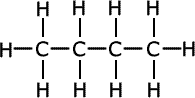
Polymers can be classified into two main categories: natural and synthetic. Natural polymers, such as cellulose, silk, and wool, are found in nature and have played a critical role in life's processes for millennia. Synthetic polymers, on the other hand, are human-made and include familiar materials like polyethylene, used in plastic bags, and polystyrene, found in disposable cups.
The physical properties of polymers, such as strength, elasticity, and resistance to chemicals and temperature, are determined by the nature of the monomers used and the way they are linked together. This allows for an incredible diversity of materials, from the tough and durable plastics used in automotive parts to the flexible and lightweight fibers in clothing.
Polymers are not only vital in the manufacturing and textile industries but also play a crucial role in the field of biomedicine. Biodegradable polymers, for example, are being developed for use in drug delivery systems and tissue engineering. The adaptability and wide range of applications of polymers make them an essential and ever-evolving part of modern science and technology.
Natural polymers
Natural polymers are macromolecules found in nature, formed by living organisms. They play a crucial role in various biological processes and are essential components of the natural world. These polymers are made up of monomers that are bonded together to form long chains, and their structure and composition are inherently designed to serve specific functions in nature.
One of the most well-known natural polymers is cellulose, the primary structural component of plants. Cellulose provides rigidity and strength to plant cell walls, enabling plants to grow tall and strong. Another vital natural polymer is DNA (Deoxyribonucleic Acid), which contains the genetic blueprint of living organisms. DNA's unique structure allows it to store and transmit genetic information, playing a fundamental role in heredity and evolution.
Proteins, another class of natural polymers, are composed of amino acids and perform a myriad of functions in living organisms. They act as enzymes to catalyze biochemical reactions, play structural roles in cells and tissues, and regulate bodily functions. For example, collagen, a protein found in connective tissues, gives skin its elasticity and strength.
Natural polymers are not only important for their biological functions but also have various applications in industries. For instance, wool and silk, both protein-based polymers, are widely used in the textile industry. The unique properties of natural polymers, such as biocompatibility and biodegradability, make them increasingly important in sustainable development and biotechnology applications.
Synthetic polymers
Synthetic polymers, unlike their natural counterparts, are human-made materials engineered for specific applications and properties. These polymers are created through a process known as polymerization, where monomers are chemically bonded into long and repeating chains. This innovation has led to the development of a vast array of materials with diverse characteristics and uses.
Synthetic polymers may be broadly sub-divided into addition and condensation polymers, although there are many other subtle forms of modifying the polymeric structures.
Addition polymers
These are formed by addition reactions of unsaturated hydrocarbons. The double bond in an unsaturated molecule opens and attaches to a neighbouring unsaturated molecule. The process continues until a large number of monomer units are joined together in a long chain.
One of the most common synthetic polymers is polyethylene, widely used in the production of plastic bags, bottles, and other packaging materials. Its popularity stems from its durability, flexibility, and resistance to moisture. Another notable synthetic polymer is polystyrene, recognized for its lightweight and insulating properties, making it ideal for use in packaging, disposable food containers, and insulation materials.
Polyvinyl chloride (PVC) is another versatile synthetic polymer used in everything from construction materials to medical devices. Its ability to be both rigid and flexible, coupled with its chemical resistance, makes it suitable for a wide range of applications. Synthetic polymers like nylon and polyester have revolutionized the textile industry, offering alternatives to natural fibers with their strength, durability, and resistance to moisture and wrinkles.
Condensation polymers
These were first developed in the mid-20th Century by joining monomer units together using condensation (addition-elimination) reactions. These polymers are dealt with in depth on the next page
Summary
The development of synthetic polymers has had a profound impact on modern society, offering materials that are integral to various industries, including automotive, aerospace, electronics, and healthcare. However, the environmental impact of these polymers, particularly their non-biodegradable nature, poses significant challenges, driving research towards more sustainable and eco-friendly alternatives.
Worked examples
Q271-01 Select the substance with the higher boiling point in each of the following pairs. Explain your reasoning:- C2H6 and C3H8
- CH3CH2OH and CH3OCH3
|
In the first pair of compounds there are no dipoles so the intermolecular forces are due to dispersion attractions only. The larger of the two molecules has the greater intermolecular force. Therefore C3H8 has the higher boiling point. In the second pair of compounds their relative masses are the same, but the alcohol, CH3CH2OH, has hydrogen bonding due to the OH group, whereas the ether (methoxymethane) has only weak dipole - dipole interactions. The alcohol, CH3CH2OH, has the higher boiling point. |
Q271-02 Which substance has the highest boiling point?
- CH4
- He
- HF
- Cl2
|
Q271-03 The very high heat of vaporization of H2O is mainly a result of
- dispersion forces
- covalent bonds
- inter-ionic attractions
- hydrogen bonding
|
The enthalpy of vaporisation is the amount of energy needed to turn one mole of a substance from liquid into vapour. It is a measure of the strength of the intermolecular forces. Water's high enthalpy of vaporisation is due to the strengthof intermolecular forces caused by hydrogen bonding. |
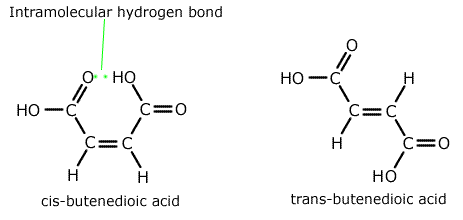
Q271-04 Explain the following statement: "Cis-butenedioic acid has a lower melting point than its isomer trans-butenedioic acid."
Answer
|
The lower melting point of the cis-butenedioic acid is indicative of the weaker intermolecular forces compared to the trans isomer. To understand why it is necessary to look at the structure of each isomer.
|
Q271-05 Arrange the following compounds in order of increasing boiling point and explain your choice by reference to the intermolecular forces involved: Bromoethene, chloroethene, ethene
Answer
|
Ethene, CH2=CH2, is a non-polar simple molecular substance with a low boiling point. However, both bromoethene, CH2=CHBr and chloroethene, CH2=CHCl are polar substances with similar polarity. Bromoethene has a much larger relative mass than chloroethene and so larger dispersion forces. This is the deciding factor, leaving the order of increasing boiling point as ethene < chloroethene < bromoethene. |
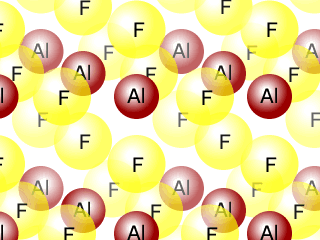
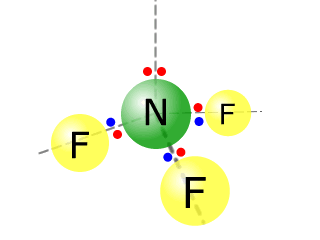
Q271-06 Aluminium fluoride AlF3 is a solid up to temperatures of 1250ºC, whereas nitrogen trifluoride is a gas above -129ºC. Describe the bonding and structure in samples of each of these substances.
Answer
|
Aluminium fluoride has a giant ionic structure, whereas nitrogen trifluoride has a simple molecular structure.
|
Q271-07 Which statements are generally true about the melting points of substances?
- I - Melting points are higher for compounds containing ions than for compounds containing molecules.
- II - A compound with a low melting point is less volatile than a compound with a higher melting point.
- III - The melting point of a compound is decreases by the presence of impurities.
- I only
- I and III only
- II and III only
- I, II and III
|
I - Melting points are higher for compounds containing ions than
for compounds containing molecules - true Correct response I and III only |
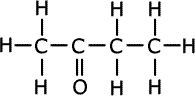
Q271-08 CH3COCH3 is the first member of the ketone homologous series. Draw the full structural formula of the next member of the homologous series and predict how its melting point compares with that of CH3COCH3
Answer
|
Members of homologous series differ by one -CH2- unit. The first member of the ketones (alkanones) is propanone, thus the second member is butanone, CH3COCH2CH3.
|
Q271-09 The compounds A, B and C have approximately the same molar mass:
| A | B | C |
| C4H10 | CH3CH2CH2OH | CH3OCH2CH3 |
When the compounds are arranged in order of increasing boiling points (lowest
boiling point first), the correct order is:
- A, C, B
- A, B, C
- B, C, A
- C, B, A
|
The three compounds belong to different homologous series, alkanes, alcohols and ethers. As they have the same relative mass we can discount the effect of dispersion forces. Alkanes are non-polar and have very low melting and boiling points. Ethers are weakly polar and have higher boiling points than alkanes. Alcohols have extensive hydrogen bonding and the highest boiling point of the three groups. The correct order of increasing boiling point is A, C, B.
|
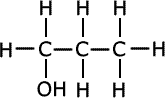
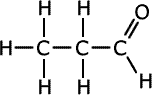
Q271-10 Identify the strongest type of intermolecular force present in each of the compounds propan-1-ol, propanal and propanoic acid. List these compounds in decreasing order of boiling point.
Answer
|
The three compounds belong to different homologous series, alcohols, aldehydes (alkanals) and carboxylic acids (alkanoic acids). Although all three compounds have different relative masses, they are not extremely different and the effect of the functional groups is greater than that of dispersion forces. In propanoic acid there is extensive hydrogen bonding and so it has the highest boiling point. The next highest boiling point is in propan-1-ol which also has hydrogen bonding. The aldehyde has only weaker dipole-dipole interactions.
|