Standard level
The 20th century has been described as the age of plastic. From the serendipidous discovery of polythene in 1898 by the German Chemist Hans von Pechmann to the equally fortunate accidental discovery of an industrial process for synthesising poly(ethene) in 1933 by the English ICI (Imperial Chemical Industry) scientists Fawcett and Gibson, synthetic polymers have become both ubiquitous and an environmental concern in everyday life.
Students should be familiar with organic structures and reactions of alkenes before studying this section.
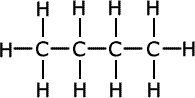
Syllabus ref: S2.4.5Structure 2.4.5 - Addition polymers form by the breaking of a double bond in each monomer.
- Represent the repeating unit of an addition polymer from given monomer structures.
Guidance
- Examples should include polymerization reactions of alkenes.
- Structures of monomers do not have to be learned, but will be provided or will need to be deduced from the polymer.
Tools and links
- Structure 3.2 - What functional groups in molecules can enable them to act as monomers for addition reactions?
- Reactivity 2.1 - Why is the atom economy 100% for an addition polymerization reaction?
Addition reactions
Addition reactions involving unsaturated hydrocarbons, such as alkenes and alkynes, are fundamental processes in organic chemistry. Unsaturated hydrocarbons are characterized by the presence of double or triple bonds between carbon atoms, which are regions of high electron density and reactivity.
In an addition reaction, the double or triple bond opens up, allowing atoms or groups of atoms to add across the bond. This process converts unsaturated hydrocarbons into saturated molecules. The most common types of addition reactions include hydrogenation, halogenation, hydrohalogenation, and hydration.
Hydrogenation involves adding hydrogen (H2) across the double or triple bond, typically in the presence of a catalyst like palladium or nickel, leading to the formation of alkanes. Halogenation is the addition of halogen atoms (such as chlorine or bromine) to the unsaturated hydrocarbon, resulting in dihalogenated products. Hydrohalogenation involves the addition of hydrogen halides (like HCl or HBr) to the hydrocarbon, while hydration adds water (H2O) across the double bond, forming alcohols.
These reactions are not only important for understanding chemical properties and reactivity of organic compounds but also have significant practical applications. For instance, the hydrogenation of vegetable oils forms margarine, a solid form of fat, and the production of important industrial chemicals and polymers often involves addition reactions of unsaturated hydrocarbons.
Addition polymers
Addition polymerization is a key chemical process where unsaturated monomers, typically alkenes or alkynes, join together without the loss of any atoms or molecules. Each monomer has a double or triple bond, which opens up to form a bond with another monomer, creating long chains or polymers. This process is integral to the production of many synthetic materials that play a vital role in modern society.
In the context of chemistry, addition polymerization may proceed via a free-radical process giving rise to products with limited structural symmetry, or using a Zeigler-Natta catalytic process that build the polymer via ionic intermediates producing highly symmetrical polymers.
This latter process allows the polymer to be designed with structures that are more crystalline, and properties that are more consistent and predictable.
Addition polymers
Society greatly benefits from addition polymerization, as it is the foundation for creating a variety of plastics and synthetic rubbers. For instance, polyethylene, formed from the polymerization of ethylene, is used in packaging, containers, and insulation. Polystyrene, another product of addition polymerization, is utilized in packaging, disposable food containers, and insulation materials. Synthetic rubbers produced through this process are essential in the automotive and manufacturing industries for tires and other rubber products.
The widespread use of these polymers highlights their importance but also raises concerns about environmental sustainability. As many of these polymers are not biodegradable, their disposal poses ecological challenges, prompting research into recyclable and biodegradable alternatives. Nevertheless, the contribution of addition polymerization to various facets of modern life is undeniable, making it a significant area of study in both chemistry and material science.
Worked examples
Q271-01 Select the substance with the higher boiling point in each of the following pairs. Explain your reasoning:- C2H6 and C3H8
- CH3CH2OH and CH3OCH3
|
In the first pair of compounds there are no dipoles so the intermolecular forces are due to dispersion attractions only. The larger of the two molecules has the greater intermolecular force. Therefore C3H8 has the higher boiling point. In the second pair of compounds their relative masses are the same, but the alcohol, CH3CH2OH, has hydrogen bonding due to the OH group, whereas the ether (methoxymethane) has only weak dipole - dipole interactions. The alcohol, CH3CH2OH, has the higher boiling point. |
Q271-02 Which substance has the highest boiling point?
- CH4
- He
- HF
- Cl2
|
Q271-03 The very high heat of vaporization of H2O is mainly a result of
- dispersion forces
- covalent bonds
- inter-ionic attractions
- hydrogen bonding
|
The enthalpy of vaporisation is the amount of energy needed to turn one mole of a substance from liquid into vapour. It is a measure of the strength of the intermolecular forces. Water's high enthalpy of vaporisation is due to the strengthof intermolecular forces caused by hydrogen bonding. |
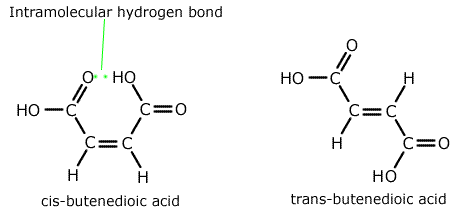
Q271-04 Explain the following statement: "Cis-butenedioic acid has a lower melting point than its isomer trans-butenedioic acid."
Answer
|
The lower melting point of the cis-butenedioic acid is indicative of the weaker intermolecular forces compared to the trans isomer. To understand why it is necessary to look at the structure of each isomer.
|
Q271-05 Arrange the following compounds in order of increasing boiling point and explain your choice by reference to the intermolecular forces involved: Bromoethene, chloroethene, ethene
Answer
|
Ethene, CH2=CH2, is a non-polar simple molecular substance with a low boiling point. However, both bromoethene, CH2=CHBr and chloroethene, CH2=CHCl are polar substances with similar polarity. Bromoethene has a much larger relative mass than chloroethene and so larger dispersion forces. This is the deciding factor, leaving the order of increasing boiling point as ethene < chloroethene < bromoethene. |
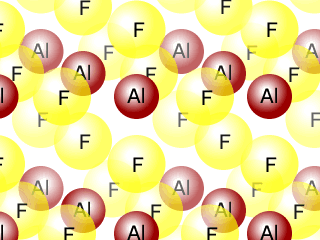
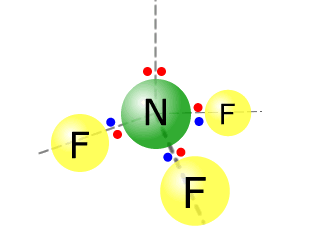
Q271-06 Aluminium fluoride AlF3 is a solid up to temperatures of 1250ºC, whereas nitrogen trifluoride is a gas above -129ºC. Describe the bonding and structure in samples of each of these substances.
Answer
|
Aluminium fluoride has a giant ionic structure, whereas nitrogen trifluoride has a simple molecular structure.
|
Q271-07 Which statements are generally true about the melting points of substances?
- I - Melting points are higher for compounds containing ions than for compounds containing molecules.
- II - A compound with a low melting point is less volatile than a compound with a higher melting point.
- III - The melting point of a compound is decreases by the presence of impurities.
- I only
- I and III only
- II and III only
- I, II and III
|
I - Melting points are higher for compounds containing ions than
for compounds containing molecules - true Correct response I and III only |
Q271-08 CH3COCH3 is the first member of the ketone homologous series. Draw the full structural formula of the next member of the homologous series and predict how its melting point compares with that of CH3COCH3
Answer
|
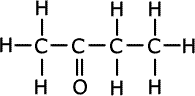
Members of homologous series differ by one -CH2- unit. The first member of the ketones (alkanones) is propanone, thus the second member is butanone, CH3COCH2CH3.
|
Q271-09 The compounds A, B and C have approximately the same molar mass:
| A | B | C |
| C4H10 | CH3CH2CH2OH | CH3OCH2CH3 |
When the compounds are arranged in order of increasing boiling points (lowest
boiling point first), the correct order is:
- A, C, B
- A, B, C
- B, C, A
- C, B, A
|
The three compounds belong to different homologous series, alkanes, alcohols and ethers. As they have the same relative mass we can discount the effect of dispersion forces. Alkanes are non-polar and have very low melting and boiling points. Ethers are weakly polar and have higher boiling points than alkanes. Alcohols have extensive hydrogen bonding and the highest boiling point of the three groups. The correct order of increasing boiling point is A, C, B.
|
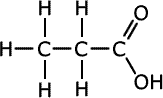
Q271-10 Identify the strongest type of intermolecular force present in each of the compounds propan-1-ol, propanal and propanoic acid. List these compounds in decreasing order of boiling point.
Answer
|
The three compounds belong to different homologous series, alcohols, aldehydes (alkanals) and carboxylic acids (alkanoic acids). Although all three compounds have different relative masses, they are not extremely different and the effect of the functional groups is greater than that of dispersion forces. In propanoic acid there is extensive hydrogen bonding and so it has the highest boiling point. The next highest boiling point is in propan-1-ol which also has hydrogen bonding. The aldehyde has only weaker dipole-dipole interactions.
|