Higher-level only
Trends in first ionization energies across the periodic table do not show an even progression; there are obvious discontinuities. These are evidence for the existence of sub-shells.
Syllabus ref: S3.1.7Structure 3.1.7 - Discontinuities occur in the trend of increasing first ionization energy across a period. (HL)
- Explain how these discontinuities provide evidence for the existence of energy sublevels.
Guidance
- Explanations should be based on the energy of the electron removed, rather than on the “special stability” of filled and half-filled sub-levels.
Tools and links
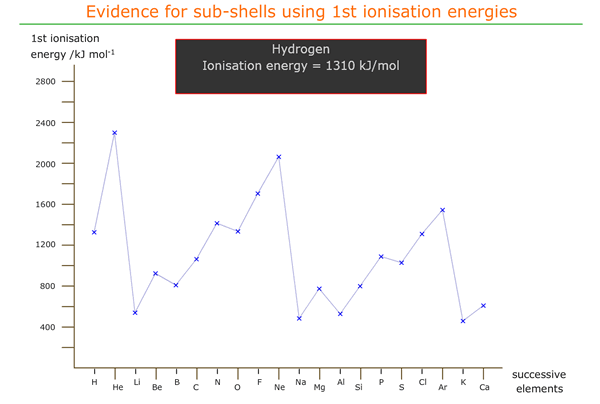
1st ionisation energies of successive elements
The first ionisation energy of an element is defined as the energy required to remove 1 mole of electrons from one mole of gaseous atoms under standard conditions.
M(g) → M+(g) + 1e
A graph of first ionisation energy against atomic number shows how the first ionisation energy varies moving from element to element in the periodic table. The outermost electron is being removed in each case and so the amount of energy needed to remove it is a function of the force holding the electron in position around the atom.
This force is dependent on two main factors and is 'fine-tuned' by a third factor.
- 1 The charge on the nucleus
- 2 The distance of the outer electron from the nucleus
- 3 Inter-electron repulsions
As the charge on the nucleus increases so the energy required to remove the electron increases. As the distance between the outermost electron and the nucleus increases so the energy required to remove it decreases.
Anomalies in 1st ionisation energy across a period
On inspection of the graph we see that there are inflexions moving across a period that seem to be contrary to the effect of increasing nuclear charge.
Moving from beryllium to boron there is a decrease in 1st ionisation energy. This can be explained as being due to the location of boron's outer electron in a 'p' orbital, which is of slightly higher energy (further from the nucleus on average).
Hence, less energy is required to remove the 2px electron from boron than the 2s electron from beryllium.
There is another inflexion from nitrogen to oxygen that must also be explained.
It appears that the energetic effect of pairing up two electrons in a 'p' orbital increases the energy of that orbital (or at least, the electrons in that orbital), meaning that less energy than expected is required to ionise one of them.
Both of these apparently anomalous inflexions also repeat across the third period, from magnesium to aluminium and from phosphorus to sulfur, with the same explanations.
Hover and click on the plots for more information.
Worked examples
Q136-01 The 1st ionisation energy of boron is slightly less than that of beryllium. This is best explained by which of the following?- The electron lost from the boron atom is in a 'p' orbital
- The electron lost from the boron atom is repelled by other electrons
- The electron lost from a boron atom is only attracted by five protons in the nucleus
- The electron lost from a boron atom is highly energetic
Answer
|
The electronic configuration of boron is 1s2 2s2 2p1 The electronic configuration of beryllium is 1s2 2s2 The 2p electron is further away from the nucleus and therefore easier to remove. Response A |
Q136-02 The first seven ionization energies of an element are 1010, 1900, 2900, 5000, 6300, 21 300 and 25 400 kJ/mole respectively. In which group of the Periodic Table is the element?
- 4
- 5
- 6
- 7
|
For these questions we look for an inflexion in the line (the point at which the slope changes dramatically). By examination of the data, we can see that there is a disproportionate jump between ionisations 5 and 6. This means that the sixth electron removed came from an orbital closer to the nucleus than the fifth. Hence there are five electrons in the outer shell, the element is from group 5. |
Q136-03 From which of the following can the value for the ionisation energy of hydrogen be calculated:
- I. The value of Planck's constant in kJ mol-1 s
- II. The value of Avogadro's constant
- III. The frequency of the convergence limit of the lines in the ultraviolet emission spectrum of hydrogen
- I only
- I and II ionly
- I and III only
- I, II and III
|
The convergence limit in the U.V. hydrogen spectrum is the transition from n = 1 to n = ∞, i.e. the ionisation frequency. The frequency is related to the energy of the wave by the equation E=hf Where h = Planck's constant This equation gives the energy for 1 transition therefore for 1 mole of transitions it must be multiplied by Avogadro's constant. The correct response is I, II and III are needed. |
Q136-04 The following graph of log ionisation energy against ionisation number is likely to be of an element in which group?
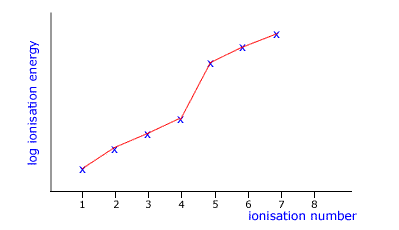
|
You can see a point of inflexion (change in direction) after the fourth ionisation. This suggests that the fifth element comes from a shell that is closer to the nucleus. For this to be the case the original element must be in group IV. |
Q136-05 The following diagram shows a section of the graph of 1st ionisation energy against atomic number. In which group of the periodic table is the element labelled A?
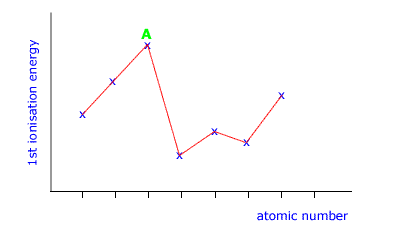
|
The peaks in this graph show those elements with the highest ionisation energy. These are the inert gases, group 18 (also called group 0) |
Q136-06 In the graph of 1st ionisation energy against atomic number, why is there a general trend towards higher ionisation energy moving across a period?
Answer
|
The increased nuclear charge moving across a period has a progressively greater attraction for the outer electron within the same shell. |
Q136-07 On which two factors does the magnitude of the electrostatic attraction between opposite charges depend?
Answer
|
The magnitude of the attracting charges and the distance between them.
|
Q136-08 Why is the energy required to remove an electron from an oxygen atom less than that required to remove an electron from a nitrogen atom?
Answer
|
The nitrogen atom has a configuration of 1s2 2s2 2p3 whereas the oxygen atom has a configuration of 1s2 2s2 2p4. Even though the electron being removed is in the same energy shell, the oxygen has the electron paired in a 'p' orbital and suffers repulsion from its pairing electron. This means that less energy is required to remove. |
Q136-09 Why is the 1st ionisation energy of potassium less than that of sodium?
|
Potassium has a larger positive nuclear charge, BUT the outer electron is in the fourth shell and much further from the nucleus. Potassium also has inner electron shells that contribute a repulsion effect, reducing the attraction of the outer electron for the nucleus. This is sometimes called 'shielding'. |
Q136-10 Why is the first ionisation energy of the 1st row transition elements fairly constant?
|
The first electron lost by the transition elements is the 4s electron. These electrons are shielded by the inner 'd' orbitals as they get filled moving across the period. The effect of adding a proton to the nucleus and adding an inner 'd' electron more or less cancel each other out. This makes the force of attraction between the outer electron and the nucleus approximately constant moving across the period. Hence the 1st ionisation energy is approximately constant. |