Higher-level only
Infra-red radiation is electromagnetic radiation with a wavelength longer than red radiation. It occupies the region of the wavelength spectrum from 700 nm to 1000 nm. The instrumentation used to analyse the effect of infra-red (IR) radiation on substances is called an infrared (IR) spectrometer.
Syllabus ref: S3.2.9Structure 3.2.9 - Infrared (IR) spectra can be used to identify the type of bond present in a molecule. (HL)
- Interpret the functional group region of an IR spectrum, using a table of characteristic frequencies (wavenumber/cm–1).
Guidance
- Include reference to the absorption of IR radiation by greenhouse gases.
- Data for interpretation of IR spectra are given in the data booklet.
Tools and links
- Structure 2.2 - What features of a molecule determine whether it is IR active or not?
- Reactivity 1.3 - What properties of a greenhouse gas determine its “global warming potential”?

Infra-red radiation
Similar in character to ordinary light only with a longer wavelength and lower energy and frequency. It is sometimes referred to as heat radiation.
The electromagnetic spectrum has rather arbitary labels assigned. The radiation with longer wavelength than visible light is called infra-red and the radiation with shorter wavelength than visible light is called ultra-violet.
IR radiation covers the electromagnetic spectrum region with a wavelength range from 1 x 10-3 metres to visible light, 7 x 10-7 metres.
Quantum theory
The microscopic world of particles is governed by laws that seem to be in conflict with our everyday experience of the world. To our limited powers of observation energy appears to be continuous, after all, a car can have any velocity from zero to its maximum. However, this only 'seems' to be that case, as we are incapable of measuring the actual stepwise absorption of energy, it's too small.
In reality, all energy changes are stepwise, this is called quantum theory.
The energy needed to change from one state to the next is called a quantum of energy.
All motion is a form of energy and molecules vibrate, rotate and translate (move from one location to another). Molecules, and the relative positions of the atoms within the molecules are in constant motion. The consequence is that these dipoles produce magnetic fields that can interact with electromagnetic radiation.
Only certain vibrations are allowed and the difference between the energy of each vibrational or rotational state corresponds to an exact quantum of energy, no more no less.
The interaction between electromagnetic radiation and matter
Electromagnetic radiation consists of an oscillating electrical field and a corresponding magnetic field (at right angles) propagating through space at the speed of light. The radiation carries energy that is directly proportional to the frequency and inversely proportional to the wavelength.
Energy = Planck's constant x Frequency
Planck's constant = 6.63 x 10-34 J s
If electromagnetic radiation carries the same energy as the difference between two quantum levels of any kind it can be absorbed, changing the quantum state of the absorber in the process.
IR radiation has just the correct energy to change the quantum levels of molecular vibrations. It can only do so, however, if this causes a change in the overall polarity of the molecule.
Care must be taken with non-polar molecules. These can also absorb IR radiation providing that doing so causes a change in polarity. This is the case, for example, of carbon dioxide. However, symmetrical molecules such as oxygen cannot absorb IR radiation, they are said to be IR inactive.
The frequency of IR radiation absorbed by a molecule depends on the bond strengths and angles. The total nuimber of absorptions depends on the number of different vibrational modes available.
The Greenhouse effect
The greenhouse effect is a natural process that warms the Earth's surface.
Sunlight Reaches Earth: Energy from the sun reaches the Earth in the form of sunlight.
Earth Absorbs and Radiates Heat: The Earth's surface absorbs the sunlight and heats up. The Earth then radiates this heat back towards space as infrared radiation.
Greenhouse Gases Trap Heat: Some gases in the atmosphere, like carbon dioxide (CO2), methane (CH4), and water vapor (H2O), absorb some of this infra-red radiation by increasing vibrational energy states in the molecules. These gases are called greenhouse gases.
Warming the Earth: By trapping heat, greenhouse gases keep the Earth's surface warmer than it would be if all the heat escaped back into space. This is similar to how the glass of a greenhouse traps heat, which is why it's called the greenhouse effect.
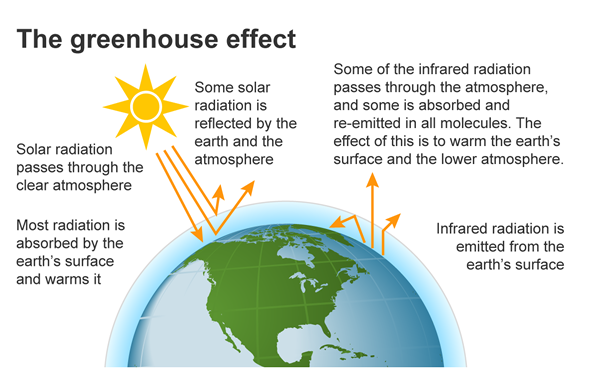
The greenhouse effect is essential for life on Earth because it keeps our planet warm enough to support ecosystems. However, human activities, like burning fossil fuels and deforestation, are increasing the concentrations of greenhouse gases, which leads to more heat being trapped and causes global warming.
The IR spectrometer
A spectrometer is an instrument used for passing IR radiation through a sample and measuring how much of the radiation is absorbed by the sample.
The IR spectrometer consists of a variable wavelength IR source, whose beam is split by rotating mirrors into two parallel beams. One beam passes through the sample, which is usually a liquid, or a mixture of a hydrocarbon 'solvent' ('Nujol') and a finely powdered solid, smeared between two discs of potassium chloride. The second, reference beam just passes through some empty potassium chloride discs.
Both beams then pass to the analyser, which, by the difference in intensity between the two beams, produces an output of frequency against radiation absorbed.
When this is displayed as a graph, it is known as an IR spectrum.
Infrared spectra
An infrared spectrum shows a series of absorptions drawn on a graph type background. The y-axis is the transmittance and the x-axis is the frequency. Traditionally the frequency is shown as a 'wavenumber'. This is the reciprocal of the wavelength in centimetres.
This convention gives a frequency scale with conveniently sized numbers. The frequency is directly proportional to the energy of the electromagnetic radiation.
IR spectra usually range from 4000 to 400 cm-1.
The energy of infrared radiation
The high energy end of the scale 4000cm-1 = wavelength 0.00025 cm = 2500 nm
The low energy end of the scale 400cm-1 = wavelength 0.0025 cm = 25000 nm
Analysing IR spectra
IR spectra are not particularly easy to analyse, nor do they give definitive information about structure. There are however, two different stages in an analysis.
- 1 Identification of absorptions
- 2 Fingerprinting
The first stage involves looking for characteristic absorptions and attempting to ascribe them to specific structural features. The second stage is usually carried out after a series of analyses leads to a possible conclusion.
Identifying absorptions
Each bond type has a different absorption frequency and a scan over a range of frequencies shows absorptions corresponding to the bonds present in the molecule.
In reality, the patterns produced in the spectra by IR absorptions are complex and only a few bond types can be identified, such as carbonyl groups C=O, and hydroxyl (alcohol) groups O-H.
Bond strength
The bond strength of an individual bond depends on the electron density between the two nuclei being held together. Groups or atoms that withdraw electrons (electronegative) tend to reduce the electron density of neighbouring groups. Consequently, a bond stretch may absorb at high frequency in one molecule and at a lower frequency in a different molecule.
|
Example: Compare the stretching frequencies of the carbonyl group in carboxylic acids and aldehydes. R-COOH, Carbonyl stretch = 1700 - 1725 cm-1 RCHO, Carbonyl stretch = 1710 - 1740 cm-1 |
Any molecular influence that increases the strength of a bond by increasing the electron density within the bond also increases the energy required to change vibrational states. More energy equates to higher frequency.
For example, a C=N double bond is about twice as strong as a C-N single bond, and the C≡N triple bond is also stronger than the double bond. The infrared stretching frequencies of these groups vary in the same order, ranging from 1100 cm-1 for C-N, to 1660 cm-1 for C=N, to 2220 cm-1 for C≡N.
Bond identification
Different bonds give rise to different stretching and bending vibrational frequencies, so a simple comparison of the absorptions recorded can be compared with known frequencies. This does not give definite identification of the molecule being analysed, but it does give clues as to the possible component parts of the molecule.
Bonds are not exactly the same strength in different types of molecule, so there is some variation in the absorbed frequency of radiation.
|
Example: An IR spectrum shows a strong absorption at a frequency of between 1680 and 1750 cm-1. What information does this provide? This frequency is indicative of a carbonyl group, C=O. The reason for the range is because the strength of the C=O bond depends also on the atoms to which it is attached. |
The fingerprint region
Use is made of the complexity of the spectrum as no two compounds have exactly the same series of absorptions. This means that a complex region of the spectrum, known as the fingerprint region, can be used to compare an unknown substance with a database of known substances.
If the unknown compound has a spectrum identical to a known spectrum, then a positive identification has been made. The fingerprint region appears at the right hand side of the diagram below between wavenumbers 500cm-1 - 1500cm-1.
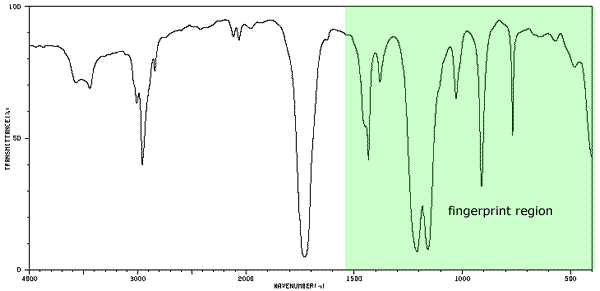
The lower 'x' axis has units of cm-1. This is known as the wavenumber, it is the reciprocal of the wavelength in centimetres. This unit of measurement is traditional for IR spectra and is used for its convenient numbers. In the diagram a strong absorption can be seen at about 3000 cm-1 corresponding to the bond between the benzene ring and a hydrogen atom. IR data
Advantages and limitations of IR spectrometry
Advantages
IR spectrometry is fast and non-destructive. It provides information about possible structural features of an unknown compound.
It is used as a supplement to other analytical techniques. The fingerprint of a molecule is practically unique and may be used for almost certain identification, if a database of similar structures is available.
Similarly, when a known compound is constructed using a new synthetic method, achievement of the 'correct' IR spectrum is evidence that the synthesis has been successful.
Limitations
It is very difficult to assign specific absorptions to definite bonds.
Worked examples
Q1134-01 Three possible compounds each have the molecular formula C3H8O. The IR spectrum of one of these isomers shows an absorption band at 1000 - 1300cm-1, But no absorption bands above 3000cm-1. State which of these three isomers corresponds to this information, giving your reasoning. IR dataAnswer
|
The absorption band at 1000 - 1300cm-1 could correspond to alcohols, ethers or esters. The fact that there is no band above 3000cm-1 eliminates alcohols. Esters have two oxygen atoms and as such cannot be involved, therefore the isomer is an ether, C2H5OCH3. |
Q1134-02 The following compounds A, B, C and D are interconverted according to the schematic below:
![]()
A, B and C are liquids at RT. Sodium metal added to each liquid produced bubbles slowly in A, but rapidly in C. The IR spectra of each of the compounds showed the following characteristic absorptions:
A, 3400cm-1; B, 1720cm-1; C, 1720 and 3100cm-1; D, 1650cm-1
By reference to the IR data explain how the absorptions can be used to identify all of the functional groups present in each of the compounds A, B C and D.Answer
|
A has an absorption at 3400cm-1, typical of alcohols. B has an absorption at 1720cm-1, typical of a carbonyl, C=O, group. C has absorptions at 1720 and 3100cm-1, typical of a carboxylic acid. D has an alkene C=C absoption at 1650cm-1 (1610-1680cm-1). |
Q1134-03 In the following schematic:
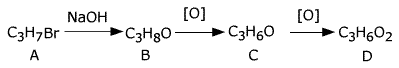
Answer
|
This absorption is typical of a carbonyl, C=O group, found at wavenumber 1680-1750cm-1 in alcohols, ketones, acids and esters. |
Q1134-04 A compound of empirical formula C4H10O, has a mass spectrum with peaks at 74 and 59, and an IR spectrum with an absorption band at 3230 - 3350cm-1. Determine the molecular formula of C, account for the peak at m/e 59 and identify the functional group causing the IR band absorption. IR data
Answer
|
The mass spectrum peak at 74 is due to the molecular ion, and is indicative of the relative molecular mass of the compound. The loss of 15 units between 74 and 59 shows loss of a CH3 group due to fragmentation. Thus the molecular formula is the same as the empirical formula, The IR absorption at 3230cm-1 is indicative of an alcohol, O-H, functional group. |
Q1134-05 The compound, C in question 4 above, can be dehydrated to give a compound D, which has an IR spectrum with a band at 1620-1680cm-1. Identify the functional group present in D. IR data
Answer|
Dehydration of alcohols results in alkenes, supported by the absorption due to C=C at wavenumber 1680cm-1. |
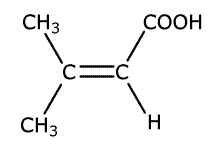
Q1134-06 The following molecule contains two important functional groups:
Answer
|
The molecule has a C=C double bond, a C=O from the COOH group and a carboxylic acid O-H gtroup. These functional groups would be expected to show absorptions at wavenumbers 1610-1680cm-1, 1680-1750cm-1 and 2500-3300cm-1 respectively. |
Q1134-07 For the compounds HOOCCH2CH3 and HOOCCHCH2, suggest how their IR spectra would differ. IR data
Answer|
The second molecule, propenoic acid, has a double bond between carbon atoms, which will show up as an absorption in the IR spectrum between 1610 and 1680cm-1. The first molecule, propanoic acid, does not have a double bond. |
Q1134-08 Compound A contains two important functional groups and has the formula (CH3)2CCHCOOH.
With the help of the IR data table identify three characteristic infrared absorption ranges corresponding to specific bonds for compound A.
Answer
|
The molecule has a C=C double bond, a C=O from the COOH group and a carboxylic acid O-H gtroup. These functional groups would be expected to show absorptions at wavenumbers 1610-1680cm-1, 1680-1750cm-1 and 2500-3300cm-1 respectively. |
Q1134-09 An unknown compound has the molecular formula C4H8O2. It gave the following IR spectrum:
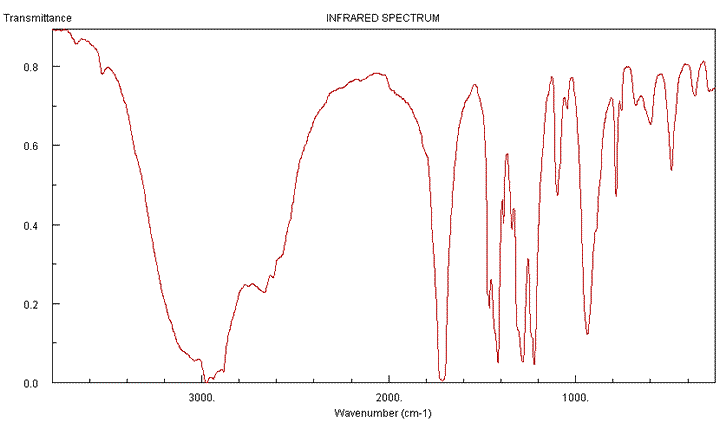
|
The very broad absorption at around about 3000cm-1 is indicative of hydrogen bonding in carboxylic acids. This is in keeping with a molecular formula, C4H8O2, having two oxygen atoms. The absorption at just under 1680cm-1 is also indicative of a C=O bond, found in carboxylic acids. We can safely surmise that the formula of the compound is C3H7COOH. This narrows the possibilities down to two isomers. |
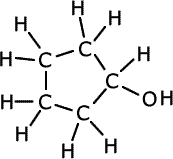
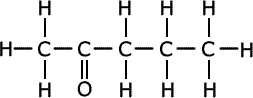
Q1134-10 The following two compounds are isomers. Suggest wavenumbers of absorptions that are present in the spectrum of each isomer and not present in the other. IR data
|
|
|
The cyclic compound at the left hand side has an OH group that is not present in its isomer. An absorption is expected at between 3230 and 3550cm-1, typical of hydrogen bonding in alcohols. The compound on the right has a carbonyl, C=O, group that is not present in its isomer. An absorption due to this functional group is expected at 1680 -1750cm-1, which will not be seen in the alcohol. |