Higher-level only
Nuclear magnetic resonance relies on the magnetic field produced by a spinning nucleus containing an odd number of nucleons (protons or neutrons). In the presence of an external magnetic field the nucleus can exhibit more than one spin state and can move between these states by the absorption of electromagnetic radiation of a specific frequency (energy).
The energy absorbed can be detected and from this information about the location (environment) of the nucleus can be deduced.
NMR is probably the most useful tool in the organic chemists arsenal for structural determination.
As organisms are mainly water (containing H atoms with an odd number of nucleons), NMR has developed into an invaluable medical diagtnostic tool, called an MRI (magnetic resonance instrument) scan.
Syllabus ref: S3.2.10Structure 3.2.10 - Proton nuclear magnetic resonance spectroscopy (1H NMR) gives information on the different chemical environments of hydrogen atoms in a molecule. (HL)
- Interpret 1H NMR spectra to deduce the structures of organic molecules from the number of signals, the chemical shifts, and the relative areas under signals (integration traces).
Guidance
Tools and links
Nuclear magnetic resonance
This tells us the number of hydrogen atoms in different environments within the molecule. As hydrogen is present in (almost) all organic compounds this technique is very useful. The pattern produced by the hydrogens is often split into finer structure, that also gives information about the number of hydrogen adjacent to the absorbing atoms.
Nuclear energy levels
Nuclei with odd numbers of nucleons (protons and neutrons) can have different energy levels. To help us differentiate these energy levels we say that they have 'spin'.
A hydrogen nucleus can have two different 'spin' states. These are designated as spin = +1/2 and spin = -1/2.
For a hydrogen nucleus to change spin states it must absorb energy (promotion) or lose energy (relaxation).
|
The magnetic field
A spinning nucleus consists of movement of electrical charge. This creates a corresponding magnetic field.
In the presence of a strong external magnetic field the hydrogen nuclei align, so that the magnetic field created by the spinning nucleus aligns with that of the external magnetic field. It is the lowest energy situation.
To change the spin state of a relaxed hydrogen nucleus, radiofrequency electromagnetic energy can be absorbed. The absorption occurs at a very specific radiofrequency, depending on the environment of the hydrogen nucleus (see below).
This absorption can be detected in an NMR spectrometer. The nuclei then relax, re-emitting the energy absorbed, in all directions, or transferring it in the form of vibrational energy to the solvent particles.
Hydrogen environments
The hydrogen nucleus aligns with the external magnetic field. It also, however, experiences other local magnetic fields caused by the electron clouds of its own electrons and those of neighbouring atoms. This means that the actual magnetic field experienced is a combination of the external field, plus all of the local magnetic fields. We say that the nucleus has a specific 'environment'.
The overall strength of the magnetic field experienced is the factor on which the radiofrequency needed to change spin states depends. Accordingly, hydrogen nuclei absorb at different radiofrequencies, depending on their local environment (assuming that we keep the external magnetic field constant). This allows analysis of the NMR absorption signals, and assignation of environments to the hydrogen nuclei that give rise to the signals.
Example: In CH3CH2OH
There are three different environments for the hydrogen atoms:
- CH3 - three hydrogen atoms in the same environment
- CH2 - two hydrogen atoms in the same environment
- OH - One hydrogen atom in a unique environment
Therefore there will be three discrete signals in the NMR spectrum
Parts per million (ppm)
The actual spectrum shows absorptions of electromagnetic radiation while the sample is held (actually rotated) in a homogenous magnetic field.
The magnetic field strength has to be maintained very stable, although in reality it is always a function of the environment. Even nearby metal shelving can affect the actual intensity of the magnetic field.
However, this problem is overcome by using a measurement method that depends on how far from an internal standard reference the absorptions occur in parts per million (ppm) of the magnetic field strength.
The internal reference is internationally agreed to be a compound that gives a strong signal close to the region where protons attached to carbon chains are likely to be found. The reference is tetramethylsilane, TMS.
Tetramethylsilane
Tetramethylsilane is a tetrahedral compound containing four methyl groups attached to a silicon atom. It is similar in structure to dimethylbutane replacing the central carbon with a silicon atom.
 |
1 Chemically very stable and unreactive. 2 Volatile liquid which can be removed easily and recovered. 3 Readily available in a pure form. 4 Sharp, strong signal as all 12 hydrogens are in the same environment. |
The signal due to tetramethylsilane, TMS, is adjusted to read zero on the spectrum. All other measurements are then taken as parts per million (ppm) shift of the absorbance when compared to this value. Each ppm shift is called 1 delta. The spectrum usually measures from 0 to 10 delta, although the instrument can usually be adjusted to read values that are higher.
 |
NMR spectrum of ethanol showing the signals measured on a scale of 0-11 ppm. The reference at zero is set using TMS.
|
Note: Knowledge of the use of TMS as a reference in NMR is not required in the 2025 syllabus
NMR Spectra
The spectrum itself is measured from the TMS zero mark at the right hand side in delta values up to delta = 10 (usually, although spectra can go much higher than this) moving left. It is possible to 'offset' the spectrum, so as to record anything that appears above delta = 10, however, the majority of organic chemistry 1H proton signals appear between delta = 0 and delta = 10.
The spectrum shows absorptions due to hydrogen atoms in specific environments.
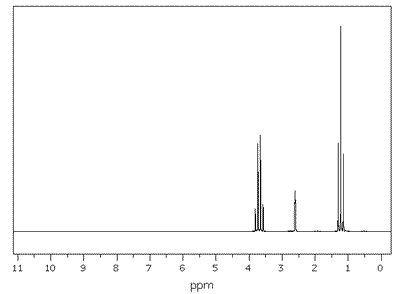
Example - The NMR spectrum of ethanol (view)

There are three major peaks at:
- delta 1.1
- delta 3.6
- delta 4.8
Corresponding to absorptions from the protons in the groups:
- CH3-
- -CH2-
- -OH
The integral
An integral to the NMR spectrum trace gives the area underneath the peak formed by the absorptions. This area (integral height) is directly proportional to the number of hydrogens causing the peak. In other words, if there are two separate groups of hydrogens in a molecule causing absorption, CH3 and CH2, then the ratio of hydrogens in these two groups is 3:2 and the height of the integral will be in the same ratio, 3:2.
The integrals are usually shown as curved lines above the actual peak being measured.

Chemical shift
The chemical shift is defined as the position of the midpoint of the absorption signal measured in ppm (delta values) from the absorption of tetramethylsilane on the NMR spectrum.
It gives us information regarding the likely environment of the absorbing protons.
The chemical shift values are recorded in tables for reference, although it is useful to remember approximate values for certain groups, for instance CH3- protons usually appear around 0.9ppm. It is important to note that these values are not set 'in stone', as they are influenced by electron withdrawing effects of neighbouring groups.
For example, when the CH3- group is attached to a benzene ring, as in methylbenzene, the absorbance appears at a chemcal shift of 2.2ppm. It is pulled downfield by the electron withdrawing influence of the benzene ring's aromatic electron system.
Any atoms or groups of atoms which withdraw electrons from their neighbouring atoms produce this effect. Hence the proton on a carboxylic acid group usually appears at ppm values of greater than 11ppm, the two electronegative oxygens exerting a strong electron withdrawing effect.
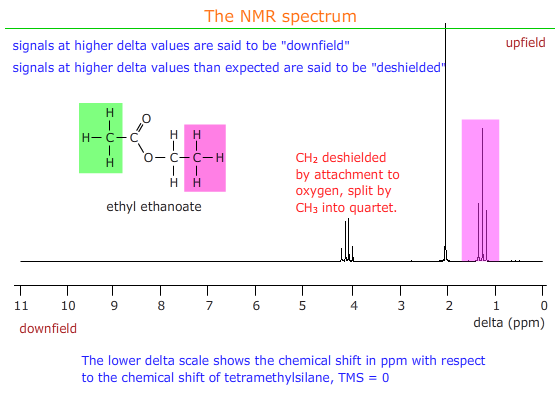
Shielding and deshielding
These two terms refer to the effect of neighbouring groups on the strength of the magnetic field experienced by a group of protons in NMR.
Electron withdrawing groups remove electron density from around protons and expose them to a stronger magnetic field - they are said to be deshielded and the signal appears downfield i.e. at higher delta values than usual for that group of protons.