Assessment tests and teaching resources
The following tests can be set for a given number of questions and the results posted to the address of choice. An analysis of incorrect responses is included. If there is no internet connection the test is fully functional, but cannot send responses by email.
| Self-test | Description |
| Atomic SL | Multiple choice questions taken from atomic theory at standard level |
| Atomic HL | Multiple choice questions taken from the whole atomic theory topic. |
| Atomic 04 | Electronic configuration in atoms and ions. |
| Atomic 05 | The aufbau principle. |
| Teaching resources | |
| ATO01 | The atomic theory (navigation top right) |
| ATO02 | The AZE convention (self-test) |
| ATO03 | First ionization energy graph |
| ATO04 | The aubau principle (configurations to z=36) |
| ATO05 | Colour absorption |
| ATO06 | Convergence limit |
| ATO07 | The hydrogen emission spectrum |
| ATO08 | Isotopic abundance and relative mass |
| ATO09 | Atomic orbitals |
| ATO10 | Successive ionization energies of potassium |
Internal video links
IB Chemistry syllabus videos (first exams May 2025), by Colourful Solutions.
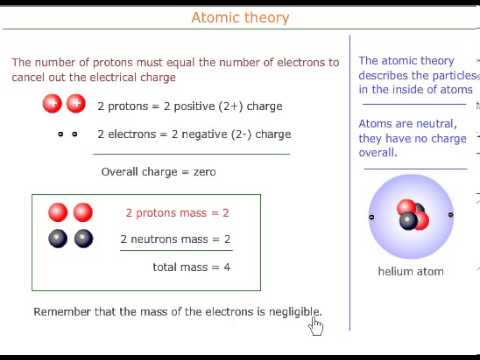
Sub-atomic particles

The aufbau principle
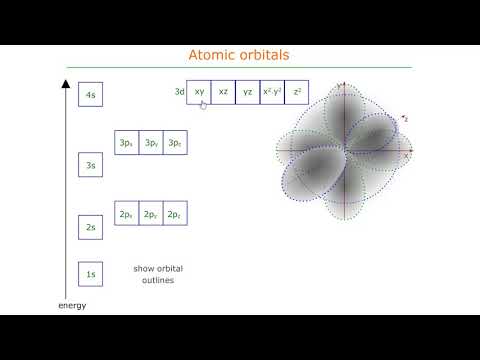
Atomic orbitals
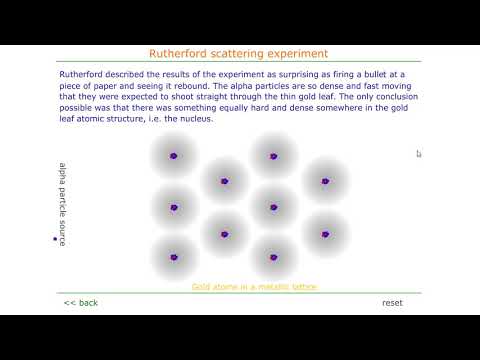
Rutherford's scattering experiment

The hydrogen visible emission spectrum
The convergence limit
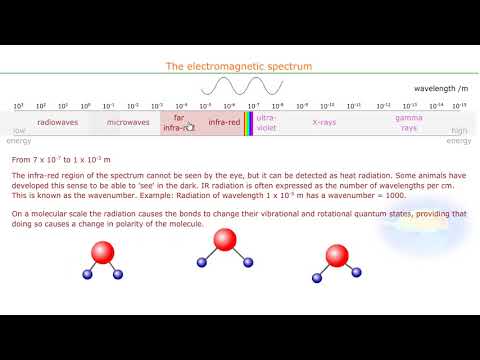
The electromagnetic spectrum
External video links
The following videos have been prepared for the IB Chemistry syllabus (first exams May 2025), by Richard Thornley, Mike Sugiyama Jones and others.

Sub-atomic particles
Mass number, atomic number and isotopes
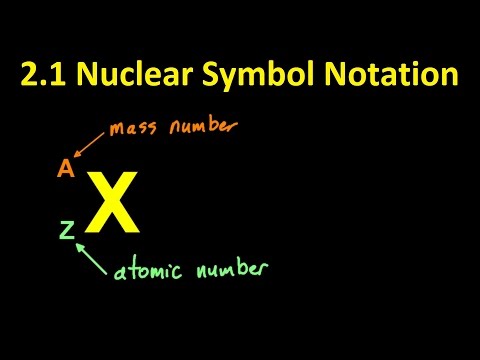
Nuclear symbol convention
Physical properties of isotopes
Non-integral relative mass calculations
The electromagnetic spectrum

Continuous and line spectra
The visible line spectrum of hydrogen

Main electron energy levels
The shapes of orbitals

Relative energy of s, p, d and f sub-shells
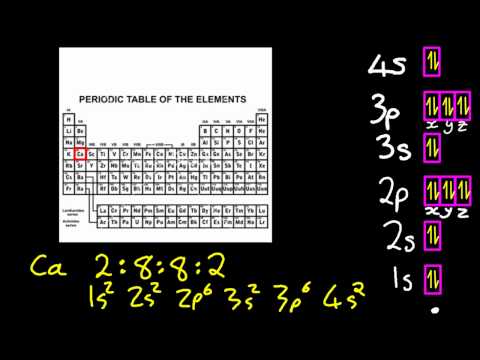
Electronic configurations up to z=36