|
The alkali metals occupy group 1 of the periodic table at the far left. They are all soft, highly reactive electropositive metals. |
Alkali metals
The alkali metals get their name from the caustic substances containing compounds of group 1 metals that were originally extracted from the ashes of burnt plants, 'al kali' in Arabic. Potassium's symbol, K, comes from the same root.

|

|
|

|

|
These metals, along with francium, occupy the far left group of the periodic table, group 1. Francium is radioactive and extremely rare and, consequently, is usually ignored.
They are all soft, silvery reactive metals that have only one electron in the outer shell. Greater shielding from the inner electrons as the group is descended makes ionisation of the atoms easier, increasing the reactivity. This is the principal feature of their chemistry.
Physical properties
The elements structure is metallic with a 'sea' of delocalised electrons surrounding a close-packed, regular array (lattice) of positive ions.
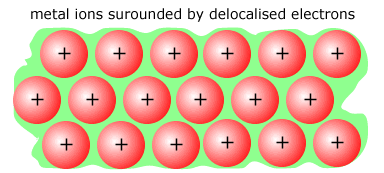 |
The force holding the structure together is the electrostatic attraction between the ions and the delocalised electrons. The smaller the ion the greater the force, as the electrons can get nearer to the nucleus. This is also called charge density, or effective nuclear charge. |
|
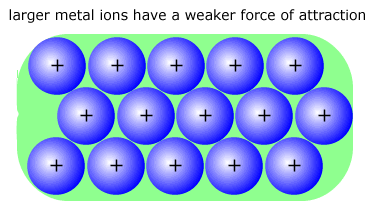
The larger metal ions on the right have a lower charge density (effective nuclear charge) and are attracted to the 'sea' of negative electrons less than smaller ions. The result of this weaker bonding is a reduction in the melting point of the alkali metals on descending the group and also a decrease in the hardness. Lithium is the alkali metal with the hardest structure and the highest melting point. |
 |
Chemical properties
The alkali metals react vigorously with oxygen, water and the halogens. The strength of reaction increases down the group. This is because the alkali metals are good reducing agents and always lose the outer shell electron when reacting, producing an ion.
The ease of electron loss increases down the group due to the increased shielding between the nucleus and the outer shell electron. The consequence is a decrease in ionisation energy (the energy needed to remove 1 mole of electrons from 1 mole of gaseous atoms) and an increase in reactivity.
The enthalpy of atomisation also decreases on descending the group (see physical properties above) favouring formation of ions. Finally, the smaller ions have a larger charge density and greater hydration enthalpy, favouring reaction. This last factor is of less importance than the previous energy changes.
The energy steps needed to form an ion in solution from an alkali metal are as follows:
| Li(s) | → | Li(g) | → | Li+(g) | → | Li+(aq) |
| atomisation | ionisation | hydration |
Comparison of actual values:
| 1st I.E. | ΔH(atom) | ΔH(hyd) | |
| Lithium | +599 | +161 | -520 |
| Sodium | +494 | +108 | -406 |
| Potassium | +418 | +90 | -322 |
Hence, for reaction of lithium, sodium and potassium with a second species, the reaction with the most exothermic change (least endothermic) is that of potassium.
| ΔH / kJ mol-1 | |
| Li(s) → Li+(aq) | +240 |
| Na(s) → Na+(aq) | +196 |
| K(s) → K+(aq) | +186 |
The values above are positive (endothermic), as no account has been taken of the energy changes of the reacting species.
The table shows that less energy is required for potassium to react than for sodium, then for lithium. This is in agreement with the observed violence of the reactions. K > Na > Li.
Reaction with oxygen
The alkali metals are all shiny, but tarnish rapidly in the air. The rate of tarnishing increases on descending the group. All of the metals react directly with oxygen producing oxides. The reactions become more energetic on descending the group.
 |
Lithium reacts to form a simple oxide, Li2O
|
 |
Sodium forms a simple oxide, but in plentiful supply of air it also forms a peroxide, Na2O2
|
 |
Potassium forms both a simple oxide, a peroxide and in plentiful supply of air (or oxygen) a superoxide, KO2 (not on the IB Syllabus)
|
The increasing reactivity down the group can be extrapolated to rubidium and caesium.
Reaction with halogens
All of the alkali metals combine directly with halogens making halide salts. The reaction gets more vigorous on descending the group.
|
Example: the reaction between sodium and chlorine: Na + Cl2 → 2NaCl Sodium + chlorine → sodium chloride |
Fluorine is the most reactive of the halogens. The most exothermic combination is therefore caesium + fluorine
Reaction with water
Perhaps one of the most well-known reactions in chemistry is the behaviour of the alkali metals in water. They all react with increasing vigour on descending the group, forming alkali metal hydroxide solutions and hydrogen gas.
 |
Lithium does not melt, but it floats while reacting due to the low density of lithium and the hydrogen gas given off. The reaction is fast and the lithium moves around the surface of the water until it has dissolved. |
 |
Sodium melts into a silvery ball and reacts very vigorously, moving rapidly around on the surface of the water. If the motion of the sodium is restricted by placing a small piece of filter paper on the surface of the water, the build up of energy is sufficient to make the sodium burst into a brilliant yellow ball of flame. |
 |
Potassium bursts into a purple/pink flame as soon as it touches the surface of the water. It melts into a silvery ball and often spits violently at the end of the reaction. |
The other alkali metals are even more aggressive in their reaction and for this reason, never carried out unless major safety precautions are taken beforehand. In all cases the reaction is as follows:
|
2M + 2H2O → 2MOH + H2 metal + water → metal hydroxide + hydrogen |
The metal hydroxide solution formed is a strong alkali (getting stronger as we down the group). Sodium hydroxide (caustic soda) is the most common of all the alkalis and is used in many areas of chemistry and industry.
Summary of group 1 - the alkali metals
| Physical properties | lithium | sodium | potassium |
| hardness |
|
||
| melting point |
|
||
| ionisation energy |
|
||
| Chemical properties | lithium | sodium | potassium |
| reactivity with air |
|
||
| rate of tarnishing |
|
||
| reactivity with halogens |
|
||
| reactivity with water |
|
||

