|
The halogens occupy the penultmate column of the periodic table. They are all reactive non-metals. |
|
Structure
The halogens are found in group 17 of the periodic table, the second group from the right hand side, just before the inert gases. They are characterised by having atoms with seven electrons in their outer energy shell. The halogens are:
 |
 |
 |
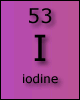 |
All four exist as simple covalent substances with only dispersion forces between their molecules. Astatine is an extremely rare, radioactive element, which also is in group 17, although not usually considered in discussions involving the halogens.
 |
The structure of solid iodine, showing the individual molecules held together by weak dispersion forces. The molecules themselves consist of two iodine atoms held by a covalent bond. This gives both of the atoms an apparent full outer shell (octet). |
Physical properties
These are typical of a simple covalent substance. The melting points (and boiling points) increase with increased relative mass of the molecules. This is in line with dispersion forces, which are proportional to the relative mass.
|
Halogen
|
 |
 |
 |
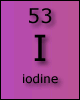 |
|
Relative mass
|
19
|
71
|
160
|
254
|
|
Boiling point/ K
|
85K
|
239K
|
332K
|
458K
|
|
State @ RT (298K)
|
pale yellow gas
|
green gas
|
dark red liquid
|
shiny grey solid
|
Chemical properties
The halogens are all reactive elements. They tend to gain electrons easiliy making them oxidising agents.
The reactivity of the halogens increases on ascending the group, with fluorine being the most reactive of all the elements, not just the halogens.
Fluorine is the strongest oxidising agent and will even oxidise water. For this reason it is very difficult to isolate - it tends to react with anything available, including glass.
Reaction with metals
The halogens combine directly with many metals making anhydrous (without water) chlorides. The oxidising nature of the halogens means that the chloride of the higher oxidation states of the metals are usually formed.
|
Fe + Cl2 → FeCl3 iron + chlorine → iron(III) chloride |
In reactions with group 1 metals, the energy of the reaction depends on both the halogen and the alkali metal used. Alkali metal reactivity increases descending the group, whereas halogen reactivity descreases on descending the group. The most vigorous reaction is between the higher halogen with the lower alkali metal.
|
Example: Which of the following pairs would give the strongest reaction. Na + Cl2 or Li + Br2? Sodium is heavier than lithium and more reactive. Bromine is heavier than chlorine and less reactive. The more reactive pair of elements is sodium and chlorine. |
Halogens as oxidising agents
The tendency of the halogens to form negative ions makes them good oxidising agents. Their oxidising power increases ascending the group.
Fluorine is too reactive to be of any practical value, but chlorine is a useful oxidising agent. Chlorine can be safely handled as a solution of chlorine in water (chlorine water). Although some of the chlorine dissociates in the solution a freshly prepared solution also contains free chlorine molecules.
The iodide ion can also behave as a reducing agent when in the presence of stronger oxidising agents. The behaviour of iodine is used in analysis reactions, such as the determination of copper 2+ ions in solution.
|
Excess potassium iodide is added to the solution to be analysed, forming iodine. 2I-(aq) + Cu2+(aq) → Cu+(s) + I2(aq) iodide ions + copper(II) ions → copper(I) ions + iodine The iodine appears as a brown colour in solution and can be analysed by titration using aqueous sodium thiosulfate. 2S2O32-(aq) + I2(aq) → S4O62-(aq) + 2I-(aq) thiosuphate ions + iodine → tetrathionate ions + iodide ions The end point of the titration is shown by the disappearance of the yellow/brown iodine. End-point judgement is helped by addition of freshly prepared starch solution just before the yellow colour has disappeared. The starch forms a deep blue/black complex with the remaining iodine, making the colour change to colourless far more obvious. |
Displacement reactions of the halogens
Iodine is a much weaker oxidising agent than chlorine or bromine and can be displaced from one of its iodide salts by addition of a more reactive halogen.
|
Cl2 + 2KI → 2KCl + I2 Chlorine + potassium iodide → potassium chloride + iodine |
The potassium ions are simply spectators to the ionic reaction:
|
Cl2 + 2I- → 2Cl- + I2 |
In this reaction electrons are being transferred from the iodide ions to the chlorine atoms. The chlorine is behaving as an oxidising agent and the iodide ions as a reducing agent. The reaction is visible as iodine imparts a red/brown colour to the solution as it is formed.
The presence of iodine can be demonstrated by the addition of a freshly prepared solution of starch, which turns a deep blue/black colour.
Summary - Physical and chemical properties of the halogens
 |
 |
 |
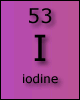 |
|
|
Reactivity
|
|
|||
|
Oxidising power
|
|
|||
|
Electronegativity
|
||||
|
Melting/boiling point
|
||||

