|
Complex ion formation is typical of transition metal chemistry. A complex ion consists of a central transition metal ion surrounded by species (atoms, molecules or ions), called ligands, which are bonded to the central metal ion by dative coordinate bonds. The whole entity may be positive, negative or even neutral, depending on the numbers and charges of the metal ion and the ligands. |
Complexes
Complexes are compounds, or ions, in which an atom (or ion) is surrounded by other species making a larger particle. The term is almost exclusively taken to mean a compound or an ion of a transition metal in which the metal ion (or atom) itself is surrounded by other atoms or ions.
This is made clearer by referring to the formulae and structures of some complexes.
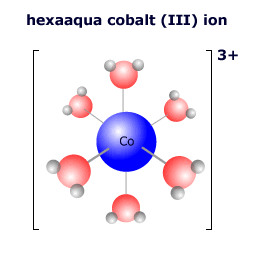 |
In the complex ion at the left there are six water molecules bonded to the central cobalt ion in an octahedral arrangement. The cobalt ion has a charge (oxidation state) of 3+. The water molecules are all neutral, so the overall charge on the complex ion is 3+ + 0 = 3+ The formula of the complex is written [Co(H2O)6]3+ |
|
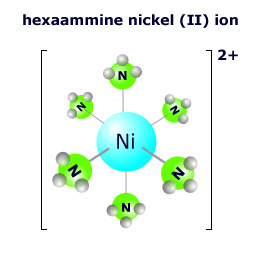
The hexaamminenickel(II) ion at the right has six ammonia molecules surrounding the central nickel 2+ ion in an octahedral arrangement. The nickel ion has an oxidation state of 2+ and each of the ammonia molecules is neutral, so the overall charge on the complex ion is: 2+ + 0 = 2+ The formula is written as [Ni(NH3)6]2+ |
 |
 |
In the complex ion at the left there are four chloride ions bonded to the central copper ion in an tetrahedral arrangement. The copper ion has a charge (oxidation state) of 2+. The chloride ions all have a charge of 1-, so the overall charge on the complex ion is 2+ + 4(1-) = 2- The formula of the complex is written [CuCl4]2-. There is no need for brackets around the chloride ions. |
Bonding in complexes
The attached ions or molecules donate an electron pair to form a covalent bond to this central atom. The bonding is called dative coordinate bonding. 'Dative' because the lone pair is donated from the bonding species.
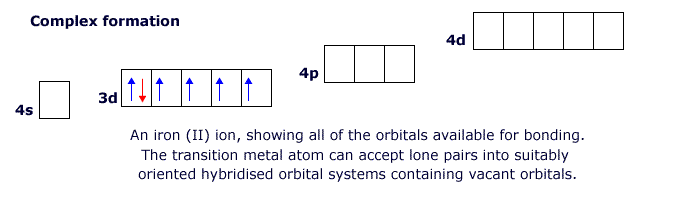
This behaviour is characteristic of the transitions metals. The first row transition elements have partially filled 'd' orbitals in the 3d level, but they also have empty 4p and 4d orbitals that can become involved in bonding. This allows the transition elements to form structures in which there are four, five or six attached species (see ligands below).
Ligands
The species that attach to the transition metal atom are called ligands (from the Latin word 'ligere' = to link). Ligands may be molecules or ions, the most common being water, hydroxide, halogens, cyanide and ammonia.
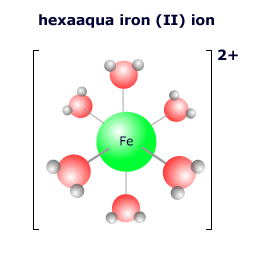 |
The ligands that attach to a transition metal atom depend on the local environment of the atom. In aqueous solution the vast excess of water molecules means that the most common transition metal complex is the hexaaqua complex ion. However, ligands are 'labile' in that they can become detached and exchange places with other ligands should the conditions change. If the concentration of chloride ions is increased, for example, the complex ion may exchange the water ligands for chloride ligands. |
Some ligands form more than one attachment to the central metal ion. These are said to be chelating, or polydentate ligands. Such ligands are more effective and are found in many biological systems, for example haemoglobin.
In the haem unit of haemoglobin, there are four electron pairs donated to the central iron atom from the same 'porphyrin' group.
Coordination number and shapes
The coordination number is the the number of attachments that all of the ligands make to the transition metal. It is the number of pairs of electrons that coordinate to the transition metal atom.
In the examples above, the hexaaqua complexes have a coordination number of 6 and the tetrachlorocuprate(II) complex has a coordination number of 4.
 |
The six water molecules bond to the central cobalt ion using a lone pair on the oxygen atom. There are six water molecules, so the coordination number is 6. The shape of this six-coordinated complex is octahedral. The ligands occupy all of the points of three-coordinate geometric axes. |
|
The copper atom has four chloride ligands bonded to the central atom. Each of the chloride ligands is bonded by means of a lone pair. The coordination number is 4. The shape of a four-coordinate complex is usually tetrahedral, although in some (rare) cases square planar complexes form. |
 |
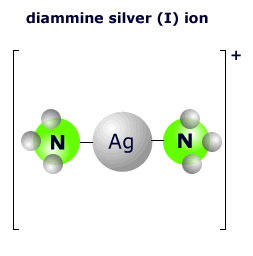 |
The two ammonia molecules bond to the central silver ion using a lone pair on the nitrogen atom. There are two nitrogen molecules, so the coordination number is 2. The shape of this two-coordinated complex is linear. The ligands are arranged at an angle of 180º. |
Nomenclature
The name of a transition metal complex must include the following:
- The name of the metal atom and its oxidation state.
- The names and numbers of the ligands
- The overall charge on the ion
The name of the metal atom actually depends on the nature of the charge on the ion. If the transition metal is part of a positive ion then the element keeps its usual name. eg. iron, copper, chromium etc, followed by the oxidation state.
However, if the ion is negative overall the transition metal atom changes its name (once again followed by the oxidation state):
| Element | part of a negative ion |
| vanadium | vanadate |
| titanium | titanate |
| chromium | chromate |
| manganese | manganate |
| iron | ferrate |
| cobalt | cobaltate |
| nickel | nickelate |
| copper | cuprate |
The names and numbers of the ligands must be stated. The ligands have special names to differentiate them from non-complexed systems.
| ligand | name |
| water | aqua |
| ammonia | ammine |
| carbon monoxide | carbonyl |
| cyanide ion | cyano |
| chloride ion | chloro |
| hydroxide ion | hydroxy |
| oxygen molecule | oxo |
| nitrogen monoxide | nitroso |
Multiplication of the ligands uses the usual di, tri, tetra, penta, hexa nomenclature.
|
Example: Name the following complex ion: [Fe(CN)6]3- The overall charge on the ion is negative, so the metal name becomes ferrate. The oxidation number needs to be worked out considering that the cyanide ion has the formula CN-. There are six cyanide ions = 6-. The overall charge on the complex ion is 3- therefore the metal ion has a charge of 3+ (3+ + 6- = 3-),i.e. oxidation state +3. There are six cyanide ions, therefore hexacyano- The complex ion's name is hexacyanoferrate(III) |
Summary
Transition metals form complex ions by coordinating ligands to the central transition metal atom, by means of donation of lone pairs from the ligand to the transition metal - dative coordinate bonding. The complexes formed have a variety of shapes and cordination numbers. Some examples are summarised below.
|
Complex
|
shape
|
ligands
|
coordination number
|
name
|
| [Fe(H2O)6]3+ | octahedral | water |
6
|
hexaaquairon(III) ion |
| [Fe(CN)6]3- | octahedral | cyanide CN- |
6
|
hexacyanoferrate(III) ion |
| [CuCl4]3- | tetrahedral | chloride Cl- |
4
|
tetrachlorocuprate(I) ion |
| [Cu(NH3)4]2+ | square planar | ammonia |
4
|
tetraamminecopper(II) ion |
| [Ag(NH3)2]+ | linear | ammonia |
2
|
diamminesilver(I) ion |
| Ni(CO)4 | tetrahedral | carbon monoxide |
4
|
tetracarbonylnickel(0) |

