|
Transition metals form compounds in which they display more than one valency. This is due to the variable oxidation states attainable by losing different numbers of '3d' electrons. |
Oxidation state
The oxidation state is defined as the apparent charge on an atom within a compound.
The oxidation state of atoms within elements is always taken to be zero. An atom increases its oxidation state (or number) by losing electrons to become more positive.
Even though in many cases the systems are not ionic, it is possible to designate oxidation states to atoms in covalent systems as if they were ionic.
Multiple oxidation states of the d-block (transition metal) elements are due to the proximity of the 4s and 3d sub shells (in terms of energy).
All transition metals exhibit a +2 oxidation state (the first electrons are removed from the 4s sub-shell) and all have other oxidation states.
The common transition metal oxidation states (Sc and Zn included for comparison)
|
Sc
|
Ti
|
V
|
Cr
|
Mn
|
Fe
|
Co
|
Ni
|
Cu
|
Zn
|
|
+1
|
|||||||||
|
+2
|
+2
|
+2
|
+2
|
+2
|
+2
|
+2
|
+2
|
+2
|
|
|
+3
|
+3
|
+3
|
+3
|
+3
|
+3
|
+3
|
+3
|
||
|
+4
|
+4
|
+4
|
|||||||
|
+5
|
|||||||||
|
+6
|
+6
|
+6
|
|||||||
|
+7
|
Electronic configuration
Variable oxidation states may be understood rather better by a consideration of the electronic configurations of the states formed.
Iron, for example has two common oxidation states, +2 and +3.
The element has the configuration [Ar]4s2 3d6. Clearly, the +2 oxidation state arises from the loss of the 4s electrons. However, loss of a further electron from the 'd' shell leaves a configuration of [Ar]4s0 3d5. This half-full set of 'd' orbitals is spherically symmetrical and has an extra degree of stability. Consequently the iron(III) state is also stable and common.
| iron | iron(II) | iron(III) |
| [Ar]4s2 3d6 | [Ar]4s0 3d6 | [Ar]4s0 3d5 |
This is not quite as simple as stated, as the nature of the environment in which the transition metal atom finds itself is also of great importance as regards stability.
An oxidation state that is stable in a solid compound may not be stable in aqueous solution and vice versa.
This is due to the crystal, or ligand field effect and depends on the molecules or ions surrounding the transition metal atom. This will be covered further in the next section.
Crystal field theory
Transition element atoms form complex ions in which the metal atom is surrounded by coordinated molecules or ions, called ligands. These ligands coordinate to the metal atom by means of electron pairs.
These electron pairs create an electrostatic field around the transition metal atom causing its 'd' orbitals to become non-degenerate (having different energy).
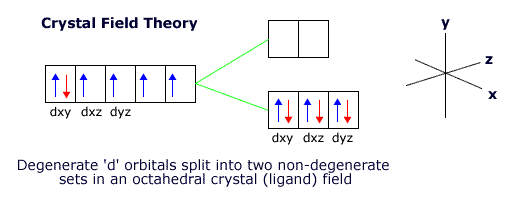
Because of the shape of the 'd' orbitals, the degenerate orbitals change into two specific groups, two of the orbitals have higher energy and the other three lower energy.
The diagram above represents the splitting that occurs when an iron(II) atom is surrounded by a strong octahedral crystal field. The six electrons in the degenerate 'd' orbitals pair up to fill the lower set of non-degenerate orbitals, saving energy in the process.
The strength of the crystal field, and the degree of splitting depends on the ligands. Powerful ligands, such as CN-, create strong fields that split the 'd' orbitals by greater amounts. Such ligands are said to be high in the spectrochemical series.
If the splitting is not very great (a ligand low in the spectrochemical series) then the electrons may still occupy the same orbitals as in the atom outside of the crystal field.
Complexes with these electronic configurations are called 'high spin', as they have more unpaired electrons.

