|
A catalyst is a substance that affects the rate of a chemical reaction and can be recovered unchanged chemically at the end of the reaction. |
|
Catalysis
Catalysts increase the rate of chemical reactions.
They may be in the same phase as the reactants (homogeneous) or in a different phase (heterogeneous).
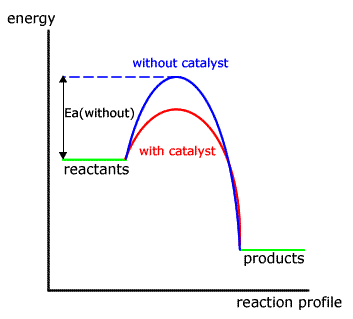
Both forms of catalyst act in a similar way, reducing the activation energy needed for a particular reaction by offering an alternative mechanism.
In the energy profile for a non-catalysed reaction the path taken is shown by the blue hump.
When a catalyst is added the different mechanism has a lower activation energy, hence there are more particles with sufficient energy to react and the reaction is faster.
In neither case is the energy of the reactants or products affected.
'd' block elements make good catalysts due to their multiple oxidation states (hence their ability to react with different species and produce a path of lower activation energy, and so allow the reaction to proceed at a faster rate).
Another possible reason for their catalytic activity could be their available empty '4d' orbitals, which allow reacting molecules to co-ordinate to the surface of the transition metal. This, in turn, weakens the bonding within the molecule encouraging reaction.
|
Examples
|
Decomposition of hydrogen peroxide
Hydrogen peroxide, H2O2, is an unstable, colourless liquid that slowly decomposes into water and oxygen in the light. For this reason it is kept in darkened bottles. The decomposition is very slow at rooom temperature.
Manganese(IV) oxide, MnO2, is an insoluble black/brown powder. When it is added to hydrogen peroxide solution, the hydrogen peroxide decomposes rapidly and the manganese dioxide catalyst can be filtered off unchanged.
This is an example of heterogeneous catalysis.
| 2H2O2 → 2H2O + O2 |
The reaction can be followed by collecting the oxygen gas evolved.
Hydrogenation of alkenes
Alkenes contain double bonds between carbon atoms. Hydrogen can be added to these bonds using hydrogen gas at 150ºC in the presence of a nickel (or platinum) catalyst.
| C2H4 + H2 → C2H6 |
This reaction, called hydrogenation, is useful in many processes, including the magarine industry. Margarine is made from vegetable oils. Vegetable oils consist of long, (C15-20) unsaturated alkyl chains linked to a propyl chain by ester linkages.
The melting temperature of the oils is increased by hydrogenation of the double bonds in the long alkyl chain. This allows the molecules to align more efficiently, increasing their bonding forces and consequently their melting temperature.
The Haber process
Developed by Fritz Haber in the early 20th century, the Haber process is the industrial manufacture of ammonia gas.
The process involves the reaction between nitrogen and hydrogen gases under pressure at moderate temperatures to produce ammonia. However, the reaction is an equilibrium and even under the most favourable conditions, less than 20% of ammonia gas is present.
Haber's adaptation to a well-known reaction, was to recycle the gaseous equilibrium mixture after rapid cooling to remove the ammonia.
The catalyst of choice in the reaction chamber is iron metal.
| N2(g) + 3H2(g) →
2NH3(g) |
The Haber process offers a means of obtaining soluble nitrogen compounds from the inert nitrogen of the air. Nitrogen compounds are of great importance in the fertilizers, drugs, explosives and many other industries.
Prior to the Haber process the majority of the worlds nitrates were obtained from 'guana', fecal deposits from sea birds, mined in great quantities on islands in the Pacific ocean. The Haber process removed this necessity.
Nowadays virtually all of the worlds supply of nitrogen compounds comes from the Haber process.
The Contact process
The contact process uses the raw materials of sulfur and air to manufacture sulfuric acid. Sulfuric acid is possibly the most important chemical in the world in terms of its incredible versatility in the manufacture of many other products.
Sulfuric acid behaves as a catalyst, an oxidising agent, a sulfonating agent, a dehydrating agent and as an acid in many diverse processes.
The contact process relies on the reaction between sulfur(IV) oxide (sulfur dioxide) and oxygen in the presence of a catalyst at 450ºC to produce sulfur trioxide.
The sulfur trixioxide is then reacted with water under special conditions to obtain sulfuric acid.
Stage 1: Sulfur from the petrochemicals industry, or volcanic mining, is burnt in air to produce sulfur dioxide
| S + O2 → SO2 |
Stage 2: The sulfur dioxide is passed with more air over a hot catalyst of vanadium(V) oxide. This catalyses the formation of sulfur trioxide (sulfur(VI) oxide)
SO2 + O2  SO3
SO3 |
(although the reaction is an equilibrium, it effectively goes 100% to the right hand side under the conditions chosen)
Stage 3: The sulfur trioxide is cooled and then dissolved in 98% sulfuric acid: 2% water. This makes the acid 100%, which can then have more water added to make 98% acid again (now with a larger volume).
The reason why water is mixed with sulfur trioxide in this way is to avoid the fine mist of sulfuric acid that could form during the extremely exothermic reaction between sulfur trioxide and water.
| SO3 + H2O → H2SO4 |
The contact process took over from the Lead Chamber process, which was less efficient and much more expensive.
Sulfuric acid is an essential chemical for any county that harbours aspirations for industry.
| Industry | product | sulfuric acid |
| Detergent | alkyl hydrogen sulfates | sulfonating agent |
| Perfumes | esters | catalyst |
| Explosives | nitro compounds | strong acid/catalyst |
| Pharmaceuticals | many | various |

